
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Fig. 5. Model of spherical electron shells of atom.Содержание книги
Поиск на нашем сайте
So each unit charge on the sphere surface is exposed to the action of force numerically equal to the value of electric field (29), generated by all other charges. Consequently total force acting on a charge of sphere will be in Q times more
The force (30) acting on a charge of sphere, it is possible to derive direct by R differentiation of formula for charged sphere energy (18). As force of "self-action" is always directed from the center it reduces total force of attraction to a nucleus (atomic core). In particular, if the external electrons shell of atom "is densely enough populated", the total force of attraction acting on each one electron of the shell is half as great! Let's notice that calculation data in tables 4, 5, 6 and 7, are based on the charged spheres model and "automatically" take into account the examined effect of electron interference in shell structure. Therefore conformity of calculations outcome with experimental data testifies to a simultaneous and constant location of electrons in spherical shells structure. So electrons are not independent particles and are not "spread" all over quantum-mechanical probability "clouds"! Keeping in mind that these electron shells are resonant formations and have the spherical form, we should mention here the corresponding mathematical solution [6]. It refers to spherical harmonics on a surface of sphere
Not striking into mathematics, we shall note, that formula (31) has three particular solutions: a zone harmonic (m=0), tesseral harmonic (0 <m <n) and sectoral harmonic (m=n). It is proved also, that any function can be expanded in spherical surface harmonics. These mathematical solutions are important with a view to probable resonance modes and degenerate energy levels.
Facts and intermediate conclusions: - The model of spherical electron shells is confirmed by experimental data (the sum of ionization potentials of the shell fits with the energy of charged sphere, simulating a shell). - The disclosed structure singularity of electron shells requires simultaneous compliance of two basic conditions: Atom structureelectrons are not independent particles; All electrons constantly and all together have to be located on spherical shells (otherwise the model of spherical electron shells would not be confirmed by experimental data).
The conclusion The principal result of this research, certainly, is the revelation of the resonant nature of electron shells of atoms which naturally arise from wave-corpuscle electron properties. It is a key concept for understanding of all other questions! Resonances in electron shells are the physical reason of electron "condensation", that is, their integration in the common resonance with "fixing" in resonance antinodes. This effect is the essence of electrons collective properties formation in shell structure. On the other hand, shell resonance is accompanied by intensive energy (mass) exchange of electrons being in antinodes. As a result there is the centrifugal force compensating force of attraction, so that electrons motionlessly "hang" above a nucleus of atom. Thus, there is an imitation of electrons movement without their real movement. And at last, electron condensation in resonant shells is the reason of the spherical shells form because "hanging" electrons follow equipotential surfaces, getting thus the "necessary" frequency to be tuned in the general resonance. These conclusions about the physical nature of electron shells are based on the analysis of experimental characteristics of atoms. Therefore fundamental results of the research can be formulated as Laws of formation of atom electron shells: 1. Electron shells of atoms are resonant formations which can be excited both on the basic frequency, and on harmonious components. Thus the principal quantum number accordingly can be integer, or fractional.
2. Many-electron atom shells have the spherical form, so the total sum of ionization potentials of a shell meets (subject to the virial theorem) the energy of the charged sphere of the same radius with electrical charge determined by the number of electrons on the shell. 3. Electrons of the atom electron shell are built in the common resonance, losing their individual properties, owing to what the electron shell obtains properties of Bose-condensate. Notes: 1. In order not to overburden the article, the description of features of electron shells of the atoms not includes the dependence on radius value (exceeds it or not the Bohr radius). This question can be examined as analogical extension by means of calculations similar to those carried out in the article. Separate consideration demands also a question of a distribution of electrons in atom over shells. We can only note that this distribution should correspond to a principle of minimum atom energy in whole. 2. Some readers of my article [1] propose for the convenience of citing and references to results of the research and in compliance with the tradition to name disclosed laws of atom electron shells formation by author’s name. Answering this proposal, I have to confess not to experience pleasure in connection with revelation of inconsistency of the modern atom theory because I as well as many other people have spent a lot of time and forces to study this theory and to overcome mistakes connected with it. As of giving author’s name to scientific discovery, it is really tradition, but it would be desirable, that it occurs as a result of discussion by scientific community.
References (in Russian) 1. Verin O.G. Basic physics of atom structure. http://www.sciteclibrary.ru/rus/catalog/pages/14155.html 2. Shpolsky E.V. Atomic physics. Vol. 2: Bases of quantum mechanics and electron shell structure of atom. 5-th ed. Ì.: “Science”. 1984. - S. 439. 3. Reference book of chemist. Edited by B.P.Nikolskiy. Vol.1. M-L.: "Chemistry", 1982. 4. Physical characteristics. Reference book. - Ì.: “Energoatomizdat”, 1991. S. 1232. 5. Yavorsky B.M., Detlaf A.A. Physics reference book. Ì.: “Science”, 1985. S.468. 6. Andre Ango. Mathematics for electro- and radio engineers., 1967. S.457.
Publishing date: February 15, 2015
|
|||||
|
Последнее изменение этой страницы: 2021-07-19; просмотров: 157; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.131.85.46 (0.009 с.) |

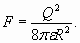 (30)
(30)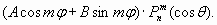 (31)
(31)


