Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Model of spherical atom electron shells
Let's look at atom electron shells from one more not less interesting point of view. The carried out analysis makes it clear that electrons in atoms belong to level-by-level arranged electron shells. Each of these layers (which we call a resonant electron shell) involves aset of electrons, incorporated in general resonance. Thus, the many-electron shell can be represented in the form of sphere with symmetrically located on it electrons (for example, on tops of the polyhedron mentally inscribed in a spherical surface). Such electron shell configuration enables at the analysis to use the simplest model - in the form of the charged sphere. Thus the structure of atom reminds "nested doll" in which center the nucleus is located, and concentric electron shells are around of it - one inside of another. How such electron shells structure effect atom properties? We shall examine it taking as example atoms of inert gases, which external electron shells "are densely populated" and really resemble charged spheres. As is known, electrostatic energy of the charged sphere is [5]
Electron shell charge Q is determined by number of electrons in the shell. Recollect also, that the ionization potential corresponds to the half of absolute value of binding energy of electron in shell structure. Hence and in view of (18), the total sum of ionization potentials of all electrons of external shell, for example, of neon atom is determined by the formula:
Really, removal of any electron from the shell (act of ionization) is accompanied by "penetration" of a corresponding part of not compensated electric field of atomic core beyond the limits of external shell that is perceived as increase of its charge. Therefore removal of each subsequent electron from the shell demands increasing energy consumption, and the total result will just correspond to the formula (19). Using formula (19), it is possible to determine each potential of ionization separately (as energy change at removal of one electron), and the total energy to represent as the sum of such energy changes
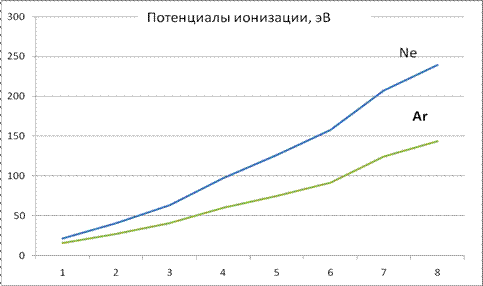
As expected, ionization potentials are proportional to the degree of ionization (formula (20) is written down in reverse order relative to degree of ionization). Electron shells, certainly, are not regularly charged spheres (especially when there is small number of electrons in the shell); nevertheless, results of experiments in whole confirm applicability of model (fig. 4). Deflections from linear dependence, obviously, are connected among other things also with processes of reorganization of electron shells after each act of ionization. As a result, the total energy of an electron shell is additionally redistributed between ionization potentials. However the total sum (20) "absorbs" all redistributions and, as in already examined case of the shell with only two electrons, enables most evidently and simply to compare results of calculations with experimental data.
|
||||
|
Последнее изменение этой страницы: 2021-07-19; просмотров: 83; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.144.98.13 (0.004 с.) |
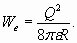 (18)
(18)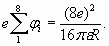 (19)
(19)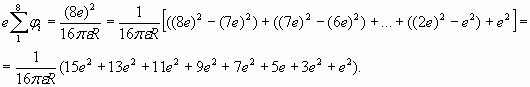 (20)
(20)