Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Fig. 4. Ionization potentials of a neon and argonСодержание книги
Поиск на нашем сайте Subject to degree of ionization Let's verify the validity of formula (19). But for this purpose it is necessary to determine radius of external electron shell as precisely as possible. For this purpose as it was already shown, the best variant is of an electron shell with only one electron. Therefore radius of the external shell of neon atom is determined on the bases of the eighth degree ionization potential
There are other methods of calculation of radius which also give close results. Having substituted concrete values in (19), we obtain
Thus, the right and the left parts of (19), really, are close to each other. Results of similar calculations for other noble gases are shown in table 4. Òàáëèöà 4
External shell of halogens atoms has only one electron less, than noble gases; therefore we can expect exact fit of calculations and experiments in this case too. However, formulas are "to be corrected" accordingly:
Calculations output and actual values are represented in table 5. Table 5
As we see, calculations output and actual values for external electron shells of halogens (as well as noble gases) correspond with each other. Whether these laws are also distinctly valid for internal atom shells? To answer this question, let us make similar calculations for the filled shells which are directly under external shells of univalent and bivalent atoms. Here also quite obvious changes in formulas are required. First we write down these formulas for atoms with only one electron on an external shell:
Similarly we can modify formulas for atoms with two electrons on an external shell:
Calculations output and actual values are represented accordingly in tables 6 and 7. Thus, made analysis convincingly shows, that electrons in atoms really concentrate layer-by-layer in spherical electron shells. Exact correspondence of calculation results to experimental data also testifies high accuracy of determining of electron shells radiuses. Table 6
Table 7
In conclusion of electron shells structure analysis it is necessary shortly talk about so-called "shielding action" on the charge of a nucleus (or atomic core) by shells electrons. What is the mechanism of "screening effect"? The model of spherical electron shells enables to specify this question. According to Gauss theorem [5] each subsequent charged sphere is exposed to the electricfield of the total charge which sits inside of this sphere. So, the charge of shells of greater radius cannot influence shells of smaller radius (according to the model of spherical electron shells, fig. 5). However in this simple scheme there is one not so obvious feature. The matter is that each charged sphere besides has “self-action”. The charges being on sphere surface are subjected to electric field equal to the half value of the field created by this charged sphere close to the external surface:
This formula can be deduced by integration of contributions of all elementary charges located on a surface of sphere.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2021-07-19; просмотров: 112; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.214 (0.009 с.) |


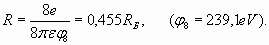 (21)
(21) (22)
(22)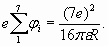 (23)
(23)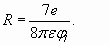 (24)
(24)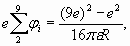 (25)
(25)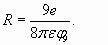 (26)
(26)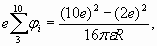 (27)
(27) (28)
(28)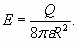 (29)
(29)