Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Samples of the typical tasks solvingСодержание книги
Поиск на нашем сайте
1. Enthalpies of the NO and NO2 formation are equal to 21,6 and 7,43 kKal/mol (respectively).Calculate heat effect of the reaction: 2NO + O2 = 2 NO2 in kJ.
∆Hf(NO) = 21,6 kCal/mole 1)∆H =2 ∙∆Hf(NO2) - 2∙∆Hf(NO)= ∆Hf(NO2)=7,43 kCal/mole = 2∙7,43 – 2 ∙21,6 = -28,34 (kCal)
∆H reaction-? 3) ∆H = -28,34 ∙4,18 =-118,46 (kJ) The answer: -118,46 kJ
2. Data: Solution: ∆Hcom(C2H2) = -1300 kJ/mole C2H2 + 2H2 = C2H6 ∆Hcom(C2H6) = -1540 kJ/mole ∆Hcom(H2) = ∆Hf(H2O) = -286 kJ/mole
∆H = (∆Hcom(C2H2) + 2∙∆Hcom(H2) – ∆H reaction-? - ∆Hcom(C2H6) =1300 - 2∙286 + 1540 = = -332 kJ The answer: -332 kJ
3. Calculate the formation heat of crystallohydrate CaCl2∙6H2O from unhydrated salt (heat of hydration) if dissolving heat of CaCl2 ∙6H2O equals + 18,02 kJ/Mole, dissolving heat of CaCl2 equals –72,9 kJ/mole. Solution: 1) Let us make up the Hess’s triangle: CaCl2 ∙6H2O
∆Hdissolving (CaCl2 ) 2) According to Hess’s triangle: ∆Hdissolv.(CaCl2 ) = ∆Hhydr. + ∆Hdissolv.(CaCl2 ∙6H2O) ∆Hhydr.= ∆Hdissol.(CaCl2 ) - ∆Hdissolv.(CaCl2 ∙6H2O) = -72,9-18,02 = -90,92 (kJ/mol). The answer: -90,92 kJ/mol
4. Calculate the change og Gibb’s energy for the process of glucose oxidation C6H12O6(s) + 6O2(g) = 6CO2(g) + 6 H2O(l) at the temperature 370C, if ∆Hcom(C6H12O6) = -2801 kJ/mole, the entropies of substances are equal to (in J/mole∙K): S(C6H12O6) = 210, S(O2) = 205, S(H2O) = 70.
Solution: 1) ∆S reaction = ∑∆Sf (prod.) - ∑∆Sf (react.) =[ 6S(CO2) + 6S(H2O)] – [S(C6H12O6) +6 S(O2) ] = 6∙ 213 + 6∙70 –(210 + 6∙205) =56(J/mole∙K) 2) ∆G = ∆H - T∙∆S = -2801-310∙ 58∙10-3 = -2881 (kJ/mole) 3) ∆G<<0 it means, that oxidation of glucose in the organism is the spontaneous process ∆H<0(exothermic process), ∆S>0, i.e. enthalpy and entropy factors favour the reaction proceeding. 5. At some temperature(T)endothermic reaction A→ B proceeds practically completely. Determine: a) sing (+or-) of ∆S reaction; b) sing of ∆G reaction B→A at the temperature T; c) ability of the reaction proceeding B→A at low temperatures.
Solution: a) B→A ∆G<0 for reaction A→ B that’s why if ∆H>0 for this reaction, ∆S >0, that follows from relationship ∆G = ∆H - T∙∆S b) ∆G>0 for reaction B→ A, ∆H<0 and ∆S<0 c) the reaction B→ A is possible at low temperature: T∙∆S is small and ∆H>T∙∆S (absolute temperature) that’s why ∆G<0.
Questions for self-control. 1. What is called “the system” in chemical thermodynamics? 2. What do you know about the classification of the “ systems”? 3 When conditions of a system state are called standard conditions? What is “the system state function”? List the state functions. 4. What energy is called “ the internal system energy (U)"? 5. What is the expression of the first law of thermodynamics? Write down the mathematical expression of the law. 6. Under what conditions the change of the internal energy of a system equals the heat has been got by the system from the surroundings. 7. What kind of process is called “ isobaric process”. What is the heat, absorbed by the system when the pressure is constant, spent on? 8. What change of the internal energy does each system tend towards? How can it be expressed? 9. What equation defines the enthalpy and its change? 10. Under what conditions does the change of enthalpy (∆H) equal the heat absorbed from the surroundings? 11. What law is the basic law of thermochemistry? Who is the author? Formulate the law. 12. What is called “the heat effect” of chemical reaction? What equations are called thermochemical? 13. What is called “the heat of formation” and “the heat of combustion”? What units are they expressed in? 14. List the consequences of the Hess’s law. For which determinations are they applied in the thermochemical calculation? 15. In the isolated systems all the spontaneous processes proceed towards the increasing of the chaos. How does the entropy change in such cases? 16. What kind of tendency is expressed by: a) enthalpy factor b) entropy factor? 17. What system state function gives the quantitative characteristic of the simultaneous influence of the enthalpy and the entropy factors. What equation can it be expressed by? 18. What kind of energy is called the “Gibbs energy” (free energy, isobaric – isotermal potential)? 19. How can the change of Gibbs energy (∆G) point at the thermodynamic possibility or impossibility of the spontaneous process proceeding? What value of ∆G determines the equilibrium state of the system? 20. In what correlation are ∆H and T∙∆S a) for the system in the equilibrium? b) if the chemical process proceed towards exothermic or endothermic reaction? 21. How can you explain the possibility of endotermic reaction proceeding. Why does this possibility increase with the increasing of the temperature? 22. What factor is the direction of a chemical reaction defined by - the enthalpy or entropy, at very low temperatures. 23. At what values of ∆H and T∙∆S,positive or negative, is only exothermic processes possible? Tasks for the self-solution 1. Calculate the value of ∆H for the reaction of the glucose convertion in the organism. a) C6H12O6(s) = 2 C2H5OH(l) + 2CO2(g) b) C6H12O6(s) + 6O2(g) = 6H2O(l) + 6CO2(g) Which of these 2 reactions gives the organism more energy? ∆Hf(C6H12O6(s)) = -1273 kJ/mole; ∆Hf(C2H5OH(l)) = -278 kJ/mole ∆Hf (CO2(g)) = -393 kJ/mole; ∆Hf(H2O(l)) = -286 kJ/mole. The answer: a) –69 kJ; b) –2801 kJ. 2. The heat of lipid, protein and carbohydrate combustion in the human body equals respectively 9,3; 4,2; 4,6 kKal/g. An average daily necessity in carbohydrates, proteins and lipids for the men-students is 451, 113, 106 g, for the women-students it is 383, 96 and 90g. What is the daily necessity in energy for the students? The answer: 3535 kCal and 3002 kCal. 3. The heat formation of H2O(l) and H2O(g) equal respectively – 286 and –242 kJ/mol. Determine the heat of water evaporation. How much heat will be lost with the evolving of 750 g of water through the skin? The answer: 44 kJ/mole; 1833 kJ. 4. Why is the trypsin protein denaturation process at 500 spontaneous? Nevertheless the reaction is endothermal, the heat effect is equal to 2735,7 kJ/mol, S of the reaction equals 8,8 kJ/mol∙K. The answer: ∆G of the reaction= - 1116,3 kJ. 5. During the reaction of 3,6 g of the iron(II) oxide with the carbon(II) oxide 28,29 kJ of the heat are evolved. Calculate standard enthalpy of the solid iron(II) oxide formation. The answer: -268,7 kJ/mol 6. With the combustion of 2 mole phosphine PH3 you can observe the formation of phosphorus (V) oxide and water with the evolving of 2440 kJ of heat. Define the standard enthalpy of phosphine formation if the standard enthalpy of phosphorus(V) oxide and water formation equal correspondingly –1528 and -286 kJ/mole. The answer: -17 kJ/mole. 7. Define the enthalpy of diamond combustion if standard enthalpy of the graphite combustion equals –393,51 kJ/mole and enthalpy of the phase transfer C(graphite)→ C(diamond) equals 1,88kJ/mol. The answer: -395,39 kJ/mole. 8. Define the enthalpy of sodium carbonate hydration if the unhydrated salt dissolving enthalpy equals –25,08 kJ/mole and dissolving enthalpy of ten-aqueous crystallohydrate is 66,88 kJ/mol. The answer: -99,96 kJ/mol. 9. Define the sing of ∆H0, ∆S0, ∆G0 (negative or positive) for the reaction AB(s) + B2(g) = AB3(s), proceeding at 298 K from the left to the right. Will the ∆G0 increase or decrease with the temperature increasing? The answer: ∆S0<0, ∆G0 <0; ∆H0<0; ∆G0 with the temperature increasing increases too. 10. Using the values of ∆H0(formation), S0 (f) and ∆G0(f) of some compounds calculate in 2 ways ∆G0298 of the reaction COCl2 = CO + Cl2 ∆H0(f),kJ/mol -223 -110,5 0 S0(f),J/mol∙K -289,2 197,4 223 ∆G0(f),kJ/mol -210,4 -137,2 0 and determine the possibility of its proceeding under standard conditions. The answer: 73,2kJ, ∆G0 >0 and the reaction is impossible under these conditions. 11. Calculate ∆H0, ∆S0, ∆G0 (at 298 K0) for reaction 2Ag(s) + Hg2Cl2(s) → 2AgCl(s) + 2Hg(l) ∆H0(f), kJ/mol 0 -264,9 -127,03 0 S0(f), J/mol∙K 42,7 196 96,11 77,4 ∆G0(f), kJ/mol 0 -210,7 -109,72 0 Prove that this reaction follows to the relationship: ∆G = ∆H - T∙∆S. Does the reaction proceed spontaneously? Does the change of enthalpy and entropy (separately) favour the spontaneous proceeding of this reaction? The answer: -8,74kJ; 10,84 kJ; 65,52 J/K; the reaction must proceed spontaneously, only entropy factor favors the reaction proceeding.
|
|||||||
|
Последнее изменение этой страницы: 2020-12-17; просмотров: 105; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.226.169.169 (0.006 с.) |

 Data: Solution:
Data: Solution:  2) 1kKal = 4,18 kJ
2) 1kKal = 4,18 kJ
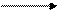
 ∆Hhydration ∆Hdissolving (CaCl2 ∙6H2O)
∆Hhydration ∆Hdissolving (CaCl2 ∙6H2O) CaCl2, H2O CaCl2
CaCl2, H2O CaCl2