Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Topic: Solutions of strong and weak electrolytesСодержание книги
Поиск на нашем сайте
In aqueous solutions strong electrolytes are completely dissociated into ions. An effective conditional molar concentration of an ion is called activity (a) of the ion (i). It equals: ai = fCi,where f – the activity coefficient. The activity coefficient for different ions changes as the concentration of the solution changes, so in concentrated solutions f< 1, and in deluted solutions it is moving towards 1. Electrolyte solution is characterized by the ionic strength of the solution(I), which can be calculated as the following: I (of the solution) = ½ ∑Ci∙Zi2, where C 1 – is the molarity of the i-ion. Zi –is the charge of i-ion in the units of the charge of the electron.
Alternatively, in aqueous solutions weak electrolytes are not completely dissociated into ions, but only a small part of them. The quantitative characteristic of this process is the degree of electrolytic dissociation (α), which depends on the strength of electrolyte and thus can be as: 0≤ α ≤ 1; for the strong electrolytes α >0,3 for the weak ones α < 0,03, and for the electrolytes of the middle strength 0,03< α <0,3. The degree of electrolytic dissociation equals
Ni Ci νi α = —— = —— = —— N C ν
Ni- the number of the molecules of the electrolyte, dissociated into ions. N- the common number of the molecules of the electrolyte in the solution (the number of the molecules of electrolyte, put into the solvent) Ci – molarity of dissociated electrolyte. C- total molarity of the electrolyte. νi – the amount of the electrolytic substance, dissociated into the ions ν - the common amount of the electrolytic substance in the solution. The degree of electrolytic dissociation depends on the number of parameters including the concentration. It makes the degree of electrolytic dissociation an unconvenient value for the quantitative description of dissociation process. The dissociation constant doesn’t depend on the concentration of the weak electrolyte in the solution. In the weak electrolyte solutions (acid HAn or base KtOH) a chemical equilibrium is established: HAn ↔ H+ + An- (1) KtOH ↔ Kt+ + OH- (2)
The equilibrium is quantitatively characterized by the dissociation constant: Ka – for weak acids HAn, Kb for weak bases KtOH. K and α are connected with each other according to the Ostvald’s law, for weak acid α2·C Ka = ———, if 1-α ≈ 1, Ka = α2·C; 1-α For weak base α2·C Kb = ———, If 1-α ≈ 1, Kb= α2·C; 1-α Therefore for a one-base weak acid
[H+]2 = Ka∙ C, [H+] = C∙α, [H+] =Ka/α
For one-acid weak base [OH-]2 = Kb·C, [OH-] = C·α, [OH-] = Kb/α
|
||||
|
Последнее изменение этой страницы: 2020-12-17; просмотров: 99; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.119.166.141 (0.008 с.) |


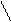
 The activity coefficient depends on the ionic strength of the solution on the following way lg f =-0,5 Zi2. I
The activity coefficient depends on the ionic strength of the solution on the following way lg f =-0,5 Zi2. I