Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Examples of typical tasks solution.Содержание книги
Поиск на нашем сайте Calculate the dissociation constant of the acetic acid if α for its 0,1 M solution equals 1,35%.
Data: Solution: C(CH3COOH) =0,1M CH3COOH ↔ CH3COO- + H+ α = 1,35% = 0,0135 Ka = α2·C; Ka = (1,35·10-2)2· 0,1 = 1,82· 10-5 The answer: Ka = 1,82· 10-5 Ka =?
6,26· 1021 molecules and ions are contained in 1L of 0,01 M solution of acetic acid. Determine the isotonic coefficient and dissociation degree of CH3COOH.
Data: Solution: Vsol.= 1L CH3COOH ↔ CH3COO- + H+
N(all particles)= 6,26· 1021 2) N(of molecules) = 6,02·1023·0,01 6,02· 1021
i=? i = ——————— = ———— = 1,04 α=? N(of molecules) 6,02·1023 4) i-1 1,04 –1 α = —— = ———— = 0,04 or 4% n-1 2-1 The answer: i = 1,04; α =4%
Calculate the ionic strength of the solution, containing 0,005 Moles of Cu(NO3)2 and 0,001 moles of Al2(SO4)3 in 1L of water, and calculate the activity of Cu2+ ions in solution (ρ = 1g/ml).
ν(Cu(NO3)2) = 0,005 M Cu(NO3)2 = Cu2+ + 2NO3- (1) ν(Al2(SO4)3) = 0,001 M Al2(SO4)3 = 2Al3+ + 3SO42-(2) ρ = 1 g/ml n
α (Cu2+) -? I(sol.)-? i=1 νi Ci = ————— V(solution)
1) According to Eq (1): ν(Cu2+) = 0,005M; ν(NO3 -) = 2∙0,005 = 0,01M 2) According to Eq (2): ν(Al3+)= 2∙0,001M; ν(SO42-) = 3∙ 0,001 = 0,003M 3) 4) α (Cu2+) = f·C(Cu2+); lgf = -0,5·Z2 I = - 0,5· 4 0,03 = 1,66; f = 0,46 α (Cu2+) = 0,46· 0,005 =0,0023 (M/L). The answer: I(sol.) = 0,03 M/L, α (Cu2+) = 0,0023 (M/L)
Determine the concentration of OH- ions and dissociation degree of ammonia in 0,01 M solution, if Kb = 1,77·10-5 Data: Solution:
1) [OH-] = 1,77·10-5 ·0,01 = 4,2 ·10-4 (M/L)
α =[OH-] / C = 4,2 ·10-4 / 0,01 = 4,2 ·10-2 The answer: [OH-] =4,2 ·10-4 (M/L) α = 4,2 ·10-2
Questions for self-control 1. What substances are called electrolytes? Tell the differences between aqueous electrolyte solutions and nonelectrolyte solutions. 2. How can you explain the electrolytic dissociation (ionization). 3. What values are the quantitative characteristics of electrolytic dissociation process (ionization). 4. What groups are all electrolytes conventionly divided by in dependence on their dissociation degrees. Give the examples. 5. What is the activity? How does it depend on the analytical concentration. 6. What is the activity coefficient, how is its value changing if the solution is being deluted. 7. What values define the activity factor for each ion and what formula can express this dependence. 8. What does it mean the ionic strength of solution and how is it determined. 9. Write down the relationship between ionic strength and molarity of diluted solutions for a) binary electrolytes of one charged and bi-charged ions (as KCl and ZnSO4; b) three – and fore-ionic electrolytes such as Na2SO4 and FeCl3. 10. How and why does the degree of dissociation of weak electrolyte depend on the introduction into its solution of the same name ion or on the dilution of the solution. 11. Why is the constant of dissociation (ionization) a more convenient characteristic of electrolyte than the degree of dissociation 12. What formula expresses the law of dilution? Define the bounds of this law application. 13. What factors does the constant of dissociation depend on? 14. In what cases Kdis = C(electrolyte) · α2. Prove the answer. 15. If the electrolyte is strong, we have [Kt+] = C(KtAn), and if the electrolyte is weak: [Kt+] = C(KtAn)· α. Why is it so? 16. How can we calculate the concentration of cation of weak electrolyte KtAn, if we know: a) dissociation constant and molarity of electrolyte b) the constant and degree of dissociation of electrolyte?
Tasks for self-solution
1. Calculate the ionic strength of K2SO4 solution whose molarity is equal to 0,02 M/L. The answer: 0,06 M/L. 2. Determine the ionic strength of the solution, containing 1,62g Ca(HCO3)2 in 250 ml of the solution. The answer: 0,28. 3. Calculate the ionic strength of solution, containing 2,08 g of BaCl2 and 5,85 g of NaCl in 500 ml of solution. The answer: 0,26. 4. Calculate the ionic strength and activity of ions in solution, containing 0,01 M of Ca(NO3)2 and 0,01 M of CaCl2 in 1L of solution. 5. Dissociation degree of acetic acid in 1N; 0,1 N; 0,01N solutions is equal to o,42%, 1,34%, and 4,25% respectively. Calculate Ka for these solutions. Prove that the constant of dissociation doesn’t depend on the concentration of the solution. 6. The dissociation constant of HNO2 equals 5,1·10-4. Calculate the degree of dissociation of its 0,01 M solution and concentration of H+ ions. The answer: 22,6%; 2,26·10-3 M/L. 7. The first dissociation constant of H3PO4 equals 7,11·10-3. Calculate the concentration of H+ -ions in 0,5 M solution if other steps of dissociation are neglected. The answer: 5,5·10-2 M/L. 8. What is the concentration of H+-ions in 1N solution of HCN if its Ka =4,9·10-10? How many grams of ions are contained in 1,5L of acid solution? The answer: 2,21·10-5 M/L; 5,75·10-4 g. 9. Determine the dissociation degree and concentration of OH- -ions in 0,1N solution of NH4OH, if Kb = 1,77·10-5. The answer:1,33%, 1,33 ·10-3 M/L. 10. How many times is the concentration of H+- ions in 1N HNO3 (α = 0,82) as high as the concentration of H+-ions in 1N H2SO4 solution (α = 0,51)? The answer: 1,6 times. 11. How much water should we add to 300 ml of 0,2 M solution of CH3COOH to double the dissociation degree of its acid?The answer: 50 ml. 12. 6,82·1018 molecules and ions are contained in 1L of 0,001M HCOOH-solution. Determine dissociation degree of formic acid. The answer: 13,3%.
Example of a test card on the topic: “The properties of electrolyte solution”
1. What is the molarity of HNO2 solution,if its dissociation degree equals 0,2? Ka= 4·10-4. a) 0,01 b) 0,01 c) 0,02 2. Choose the right formula for the calculation of Ka for acid with α<<0,03. a) α2· C b) α2· C c) α· C ——— 1 – α 3. What is the ionic strength of C-molarity solution of NaCl equal to? a) 0,5 C b) 1C c) 2C
4. What factors does the dissociation constant depend on? a) temperature b)concentration c) solubility in water.
Calculate Ka of acetic acid, if dissociation degree of 0,01 M solution equals 2% at 250C. a) 1,35·10-4 b) 1,75·10-5 c) 9·10-6 The answers to the tasks on the topic “The properties of electrolyte solutions”
Number of question 1 2 3 4 5 Code of answer b b b a c Class 3.
|
||
|
Последнее изменение этой страницы: 2020-12-17; просмотров: 162; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.108 (0.008 с.) |



 C(CH3COOH) =0,01 1) υ (CH3COOH) = C · Vsol. = 0,01M
C(CH3COOH) =0,01 1) υ (CH3COOH) = C · Vsol. = 0,01M 3) N(all particles) 6,02· 1021
3) N(all particles) 6,02· 1021 Data: Solution:
Data: Solution: I = ½ ∑ Ci∙ Zi2
I = ½ ∑ Ci∙ Zi2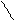




 I(solution) = ½ (0,005∙ 22 + 0,001(-1)2 + 0,002∙32 + 0,003(-2)2) = 0,03 (m/L)
I(solution) = ½ (0,005∙ 22 + 0,001(-1)2 + 0,002∙32 + 0,003(-2)2) = 0,03 (m/L) C(NH3) = 0,01 M/L NH3 + H2O = NH4+ + OH-
C(NH3) = 0,01 M/L NH3 + H2O = NH4+ + OH-


 Kb = 1,77·10-5 Kb
Kb = 1,77·10-5 Kb [OH-]= Kb·C; α = ——;
[OH-]= Kb·C; α = ——;

 [OH-] -? α -? C
[OH-] -? α -? C

 2) α = 1,77·10-5/0,01 = 4,2· 10-2 or
2) α = 1,77·10-5/0,01 = 4,2· 10-2 or