Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
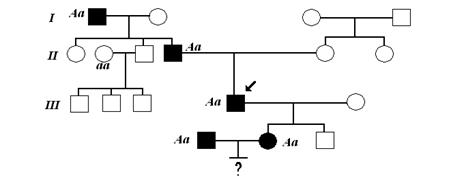
Эффективна подача кислорода через маску
Нет Да.- Делать: Целевое Sp02 - 92- 96%
Делать: Использовать N-95/ FFP-2 и другие методы , r Да Рассмотреть: HFNC .-
Делать: Надлежащий инфекционный Инфекционного контроля Не переносит 1 r Нет в наличии Контроль
Делать: Ограничить персонал в палате
&. Рассмотреть если доступен Видеоларингоскоп -- - Показания к интубации трахеи
Нет 1 r & Рассмотреть: HFNC Делать: тщательный мониторинг (S) Не делать: отсрочка интубации Трахеи при ихудшении --- (S) Не делать: Отсрочка интубации при ухудшении
Поиск Литературы COVID-19
Суммация данных
 
.....,._
1 1 - 1. 1
Распространение Ускоренный обзор и публикация
J]
Обсуждение в рабочей группе
References 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C, (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med; doi:10.1056/NEJMoa2002032. 2. European Centre for Disease Prevention and Control (2020, March 4) Situation update worldwide, 4 March 2020. In: Editor (ed)^(eds) Book Situation update worldwide, 4 March 2020. City, pp.
3. Alhazzani W, Lewis K, Jaeschke R, Rochwerg B, Moller MH, Evans L, Wilson KC, Patel S, Coopersmith CM, Cecconi M, Guyatt G, Akl EA, (2018) Conflicts of interest disclosure forms and management in critical care clinical practice guidelines. Intensive Care Med 44: 1691-1698 4. Vandvik PO, Alhazzani W, Moller MH, (2018) Understanding conflicts of interest. Intensive Care Med 44: 1738-1740 5. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schunemann HJ, (2011) GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 64: 395-400 6. Akl EA, Johnston BC, Alonso-Coello P, Neumann I, Ebrahim S, Briel M, Cook DJ, Guyatt GH, (2013) Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One 8: e57132 7. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW, (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924-926 8. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH, (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64: 401-406 9. Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R, Brozek J, Vist G, Rind D, Akl EA, Schunemann HJ, (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66: 719-725 10. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B, Alonso-Coello P, Post PN, Busse JW, Glasziou P, Christensen R, Schunemann HJ, (2013) GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 66: 158-172 11. Schunemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, Brignardello-Petersen R, Neumann I, Falavigna M, Alhazzani W, Santesso N, Zhang Y, Meerpohl JJ, Morgan RL, Rochwerg B, Darzi A, Rojas MX, Carrasco-Labra A, Adi Y, AlRayees Z, Riva J, Bollig C, Moore A, Yepes-Nunez JJ, Cuello C, Waziry R, Akl EA, (2017) GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol 81: 101-110 12. Wu Z, McGoogan JM, (2020) Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA; doi: 10.1001/jama.2020.2648 13. Livingston E, Bucher K, (2020) Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 14. Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ, (2013) Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 9: e1003205 15. Twu SJ, Chen TJ, Chen CJ, Olsen SJ, Lee LT, Fisk T, Hsu KH, Chang SC, Chen KT, Chiang IH, Wu YC, Wu JS, Dowell SF, (2003) Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis 9: 718-720
16. World Health Organization (2020, March 14) Clinical management of severe acute respiratory infection (sari) when covid-19 disease is suspected. In: Editor (ed)^(eds) Book Clinical management of severe acute respiratory infection (sari) when covid-19 disease is suspected. City, pp. 17. Cabrini L, Landoni G, Zangrillo A, (2020) Minimise nosocomial spread of 2019-nCoV when treating acute respiratory failure. Lancet 395: 685 18. Yam LY, Chen RC, Zhong NS, (2003) SARS: ventilatory and intensive care. Respirology 8 Suppl: S31-35
19. Qian H, Li Y, Sun H, Nielsen PV, Huang X, Zheng X, (2010) Particle removal efficiency of the portable HEPA air cleaner in a simulated hospital ward. Building Simulation 3: 215-224 20. Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE, (2016) Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ 188: 567-574 21. Radonovich LJ, Jr., Simberkoff MS, Bessesen MT, Brown AC, Cummings DAT, Gaydos CA, Los JG, Krosche AE, Gibert CL, Gorse GJ, Nyquist AC, Reich NG, Rodriguez-Barradas MC, Price CS, Perl TM, Res Pi, (2019) N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel: A Randomized Clinical Trial. JAMA 322: 824-833 22. Loeb M, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, Webby R, Smieja M, Earn DJ, Chong S, Webb A, Walter SD, (2009) Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 302: 1865-1871 23. MacIntyre CR, Wang Q, Cauchemez S, Seale H, Dwyer DE, Yang P, Shi W, Gao Z, Pang X, Zhang Y, Wang X, Duan W, Rahman B, Ferguson N, (2011) A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses 5: 170-179 24. MacIntyre CR, Wang Q, Rahman B, Seale H, Ridda I, Gao Z, Yang P, Shi W, Pang X, Zhang Y, Moa A, Dwyer DE, (2014) Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med 62: 1-7 25. MacIntyre CR, Wang Q, Seale H, Yang P, Shi W, Gao Z, Rahman B, Zhang Y, Wang X, Newall AT, Heywood A, Dwyer DE, (2013) A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med 187: 960-966 26. Long Y, Hu T, Liu L, Chen R, Guo Q, Yang L, Cheng Y, Huang J, Du L, (2020) Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med; doi: 10.1111/jebm.12381 27. Tuite AR, Fisman DN, (2020) Reporting, Epidemic Growth, and Reproduction Numbers for the 2019 Novel Coronavirus (2019-nCoV) Epidemic. Ann Intern Med; doi: 10.7326/M20-0358 28. Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L, (2014) Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 14: 480 29. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J, (2012) Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 7: e35797 30. Lewis SR, Butler AR, Parker J, Cook TM, Schofield-Robinson OJ, Smith AF, (2017) Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation: a Cochrane Systematic Review. Br J Anaesth 119: 369-383 31. Lewis SR, Butler AR, Parker J, Cook TM, Smith AF, (2016) Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev 11: CD011136 32. Russell TM, Hormis A, Rotherham NHSFT, (2018) Should the Glidescope video laryngoscope be used first line for all oral intubations or only in those with a difficult airway? A review of current literature. J Perioper Pract 28: 322-333
33. Center of Disease Control (2020, February 14) Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). In: Editor (ed)^(eds) Book Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). City, pp. 34. Linton NM, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov AR, Jung SM, Yuan B, Kinoshita R, Nishiura H, (2020) Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J Clin Med;doi: 10.3390/jcm9020538 35. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brunink S, Schneider J, Schmidt ML, Mulders D, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C, (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill;doi: 10.2807/1560-7917.ES.2020.25.3.2000045 36. Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, Ng DYM, Wan CKC, Yang P, Wang Q, Peiris M, Poon LLM, (2020) Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem; doi: 10.1093/clinchem/hvaa029 37. Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H, Ma S, Chen X, Long B, Si G, Yu H, Jiang L, Yang X, Shi Y, Yang Z, (2020) Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis;doi: 10.1016/j.ijid.2020.02.050 38. Yam WC, Chan KH, Poon LL, Guan Y, Yuen KY, Seto WH, Peiris JS, (2003) Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J Clin Microbiol 41: 4521-4524 39. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L, (2020) Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology;doi: 10.1148/radiol.2020200642 40. Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, Li Q, Gu S, Xu T, Li Y, Lu B, Zhan Q, (2020) Co- infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg Infect Dis;doi: 10.3201/eid2606.200299
41. Chan PK, To WK, Ng KC, Lam RK, Ng TK, Chan RC, Wu A, Yu WC, Lee N, Hui DS, Lai ST, Hon EK, Li CK, Sung JJ, Tam JS, (2004) Laboratory diagnosis of SARS. Emerg Infect Dis 10: 825- 831 42. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y, (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med;doi: 10.1016/S2213-2600(20)30079-5 43. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z, (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA;doi: 10.1001/jama.2020.1585 44. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B, (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506 45. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B, (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet;doi: 10.1016/S0140-6736(20)30566-3 46. Ruan Q, Yang K, Wang W, Jiang L, Song J, (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med;doi: 10.1007/s00134-020-05991-x
47. Bednarczyk JM, Fridfinnson JA, Kumar A, Blanchard L, Rabbani R, Bell D, Funk D, Turgeon AF, Abou-Setta AM, Zarychanski R, (2017) Incorporating Dynamic Assessment of Fluid Responsiveness Into Goal-Directed Therapy: A Systematic Review and Meta-Analysis. Crit Care Med 45: 1538-1545 48. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT, (2016) Will This Hemodynamically Unstable Patient Respond to a Bolus of Intravenous Fluids? JAMA 316: 1298- 1309 49. Pan J, Peng M, Liao C, Hu X, Wang A, Li X, (2019) Relative efficacy and safety of early lactate clearance-guided therapy resuscitation in patients with sepsis: A meta-analysis. Medicine (Baltimore) 98: e14453 50. Hernandez G, Ospina-Tascon GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegria L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernandez P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J, The ASI, the Latin America Intensive Care N, Hernandez G, Ospina-Tascon G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegria L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernandez P, Barahona D, Cavalcanti AB, Bakker J, Hernandez G, Alegria L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavez N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, Gonzalez H, Arancibia JM, Munoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Munoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpan B, Fasce F, Luengo C, Medel N, Cortes C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, Gonzalez MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudin A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, Garcia F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garces P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Perez V, Delgado G, Lopez A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderon A, Paredes G, Barberan JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernan Portilla A, Davila H, Mora JA, Calderon LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL, (2019) Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 321: 654-664
51. Meyhoff TS, Moller MH, Hjortrup PB, Cronhjort M, Perner A, Wetterslev J, (2020) Lower versus higher fluid volumes during initial management of sepsis: a systematic review with meta-analysis and trial sequential analysis. Chest;doi: 10.1016/j.chest.2019.11.050 52. Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E, (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta- analysis. Intensive Care Med 43: 155-170 53. Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM, Group FT, (2011) Mortality after fluid bolus in African children with severe infection. N Engl J Med 364: 2483-2495 54. Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, Roberts I, (2018) Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev 8: CD000567 55. Antequera Martin AM, Barea Mendoza JA, Muriel A, Saez I, Chico-Fernandez M, Estrada-Lorenzo JM, Plana MN, (2019) Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev 7: CD012247
56. Moller MH, Claudius C, Junttila E, Haney M, Oscarsson-Tibblin A, Haavind A, Perner A, (2016) Scandinavian SSAI clinical practice guideline on choice of first-line vasopressor for patients with acute circulatory failure. Acta Anaesthesiol Scand 60: 1347-1366 57. Gamper G, Havel C, Arrich J, Losert H, Pace NL, Mullner M, Herkner H, (2016) Vasopressors for hypotensive shock. Cochrane Database Syst Rev 2: CD003709 58. Honarmand K, Um KJ, Belley-Cote EP, Alhazzani W, Farley C, Fernando SM, Fiest K, Grey D, Hajdini E, Herridge M, Hrymak C, Moller MH, Kanji S, Lamontagne F, Lauzier F, Mehta S, Paunovic B, Singal R, Tsang JL, Wynne C, Rochwerg B, (2020) Canadian Critical Care Society clinical practice guideline: The use of vasopressin and vasopressin analogues in critically ill adults with distributive shock. Can J Anaesth 67: 369-376 59. McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, Lamontagne F, Healey JS, Whitlock RP, Belley-Cote EP, (2018) Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA 319: 1889-1900 60. Lamontagne F, Day AG, Meade MO, Cook DJ, Guyatt GH, Hylands M, Radermacher P, Chretien JM, Beaudoin N, Hebert P, D'Aragon F, Meziani F, Asfar P, (2018) Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 44: 12-21 61. Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, Camsooksai J, Darnell R, Gordon AC, Henry D, Hudson N, Mason AJ, Saull M, Whitman C, Young JD, Rowan KM, Mouncey PR, trial i, (2020) Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA;doi: 10.1001/jama.2020.0930 62. Moller MH, Granholm A, Junttila E, Haney M, Oscarsson-Tibblin A, Haavind A, Laake JH, Wilkman E, Sverrisson KO, Perner A, (2018) Scandinavian SSAI clinical practice guideline on choice of inotropic agent for patients with acute circulatory failure. Acta Anaesthesiol Scand 62: 420-450 63. Rygard SL, Butler E, Granholm A, Moller MH, Cohen J, Finfer S, Perner A, Myburgh J, Venkatesh B, Delaney A, (2018) Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 44: 1003-1016 64. Lamontagne F, Rochwerg B, Lytvyn L, Guyatt GH, Moller MH, Annane D, Kho ME, Adhikari NKJ, Machado F, Vandvik PO, Dodek P, Leboeuf R, Briel M, Hashmi M, Camsooksai J, Shankar-Hari M, Baraki MK, Fugate K, Chua S, Marti C, Cohen D, Botton E, Agoritsas T, Siemieniuk RAC, (2018) Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ 362: k3284 65. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L, (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507-513 66. van den Boom W, Hoy M, Sankaran J, Liu M, Chahed H, Feng M, See KC, (2020) The Search for Optimal Oxygen Saturation Targets in Critically Ill Patients: Observational Data From Large ICU Databases. Chest 157: 566-573 67. Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schunemann HJ, Neary JD, Alhazzani W, (2018) Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 391: 1693-1705 68. Siemieniuk RAC, Chu DK, Kim LH, Guell-Rous MR, Alhazzani W, Soccal PM, Karanicolas PJ, Farhoumand PD, Siemieniuk JLK, Satia I, Irusen EM, Refaat MM, Mikita JS, Smith M, Cohen DN, Vandvik PO, Agoritsas T, Lytvyn L, Guyatt GH, (2018) Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ 363: k4169
69. Investigators I-R, the A, New Zealand Intensive Care Society Clinical Trials G, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R, Young P, (2019) Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. N Engl J Med
70. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G, Investigators L, Network RR, Investigators L, Network RR, (2020) Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 382: 999 71. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian JC, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Beduneau G, Deletage-Metreau C, Richard JC, Brochard L, Robert R, Group FS, Network R, (2015) High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 372: 2185-2196 72. Ni YN, Luo J, Yu H, Liu D, Liang BM, Liang ZA, (2018) The effect of high-flow nasal cannula in reducing the mortality and the rate of endotracheal intubation when used before mechanical ventilation compared with conventional oxygen therapy and noninvasive positive pressure ventilation. A systematic review and meta-analysis. Am J Emerg Med 36: 226-233 73. Ou X, Hua Y, Liu J, Gong C, Zhao W, (2017) Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ 189: E260-E267 74. Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, Mekontso-Dessap A, Schreiber A, Azoulay E, Mercat A, Demoule A, Lemiale V, Pesenti A, Riviello ED, Mauri T, Mancebo J, Brochard L, Burns K, (2019) High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med 45: 563-572 75. Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, Gravel D, Henry B, Lapinsky S, Loeb M, McDonald LC, Ofner M, Paton S, Reynolds D, Scales D, Shen S, Simor A, Stewart T, Vearncombe M, Zoutman D, Green K, (2010) Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One 5: e10717 76. Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, Simor AE, Stewart TE, (2004) Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med 169: 1198-1202 77. Leung CCH, Joynt GM, Gomersall CD, Wong WT, Lee A, Ling L, Chan PKS, Lui PCW, Tsoi PCY, Ling CM, Hui M, (2019) Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect 101: 84-87 78. Cheung JC, Ho LT, Cheng JV, Cham EYK, Lam KN, (2020) Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med;doi: 10.1016/S2213-2600(20)30084- 9 79. Alraddadi BM, Qushmaq I, Al-Hameed FM, Mandourah Y, Almekhlafi GA, Jose J, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Shalhoub S, Abdulmomen A, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Sadat M, Tlayjeh H, Merson L, Hayden FG, Fowler RA, Arabi YM, Saudi Critical Care Trials G, (2019) Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses 13: 382-390 80. Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, Hawa H, Alothman A, Khaldi A, Al Raiy B, (2014) Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 160: 389-397 81. Zayed Y, Banifadel M, Barbarawi M, Kheiri B, Chahine A, Rashdan L, Haykal T, Samji V, Armstrong E, Bachuwa G, Al-Sanouri I, Seedahmed E, Hernandez DA, (2019) Noninvasive Oxygenation Strategies in Immunocompromised Patients With Acute Hypoxemic Respiratory Failure: A Pairwise and Network Meta-Analysis of Randomized Controlled Trials. J Intensive Care Med: 885066619844713
82. Xu XP, Zhang XC, Hu SL, Xu JY, Xie JF, Liu SQ, Liu L, Huang YZ, Guo FM, Yang Y, Qiu HB, (2017) Noninvasive Ventilation in Acute Hypoxemic Nonhypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Crit Care Med 45: e727-e733 83. Wang T, Zhang L, Luo K, He J, Ma Y, Li Z, Zhao N, Xu Q, Li Y, Yu X, (2016) Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. BMC Pulm Med 16: 129 84. Esquinas AM, Egbert Pravinkumar S, Scala R, Gay P, Soroksky A, Girault C, Han F, Hui DS, Papadakos PJ, Ambrosino N, International NIVN, (2014) Noninvasive mechanical ventilation in high-risk pulmonary infections: a clinical review. Eur Respir Rev 23: 427-438 85. Brochard L, Lefebvre JC, Cordioli RL, Akoumianaki E, Richard JC, (2014) Noninvasive ventilation for patients with hypoxemic acute respiratory failure. Semin Respir Crit Care Med 35: 492-500 86. Slutsky AS, Ranieri VM, (2013) Ventilator-induced lung injury. N Engl J Med 369: 2126-2136 87. Hui DSC, Zumla A, (2019) Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect Dis Clin North Am 33: 869-889 88. Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, et al., (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333: 817-822 89. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi PMOTSC, Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S, Raoof SMOTTF, (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J; doi: 10.1183/13993003.02426-2016 90. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP, (2016) Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 315: 2435-2441 91. Hui DS, Chow BK, Lo T, Ng SS, Ko FW, Gin T, Chan MTV, (2015) Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest 147: 1336-1343 92. Walkey AJ, Goligher EC, Del Sorbo L, Hodgson CL, Adhikari NKJ, Wunsch H, Meade MO, Uleryk E, Hess D, Talmor DS, Thompson BT, Brower RG, Fan E, (2017) Low Tidal Volume versus Non- Volume-Limited Strategies for Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 14: S271-S279 93. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR, (1998) Effect of a protective- ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347- 354 94. Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A, (2006) A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 34: 1311-1318 95. Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301-1308 96. Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr., Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S, (1999) Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27: 1492-1498 97. Orme J, Jr., Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, Crapo RO, Weaver LK, (2003) Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 167: 690-694 98. Wu G, Lu B, (1998) [The application of low tidal volume pressure-controlled ventilation in patients with acute respiratory distress syndrome]. Hunan Yi Ke Da Xue Xue Bao 23: 57-58
99. Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ, American Thoracic Society ESoICM, Society of Critical Care M, (2017) An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 195: 1253-1263 100. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP, (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43: 304-377 101. Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L, (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32: 1515-1522 102. Yasuda H, Nishimura T, Kamo T, Sanui M, Nango E, Abe T, Takebayashi T, Lefor AK, Hashimoto S, (2017) Optimal plateau pressure for patients with acute respiratory distress syndrome: a protocol for a systematic review and meta-analysis with meta-regression. BMJ Open 7: e015091 103. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart L, Blood Institute ACTN, (2004) Higher versus lower positive end- expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327- 336 104. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE, Lung Open Ventilation Study I, (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299: 637-645 105. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L, Expiratory Pressure Study G, (2008) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299: 646-655 106. Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G, (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303: 865-873 107. Guo L, Xie J, Huang Y, Pan C, Yang Y, Qiu H, Liu L, (2018) Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiol 18: 172 108. Walkey AJ, Del Sorbo L, Hodgson CL, Adhikari NKJ, Wunsch H, Meade MO, Uleryk E, Hess D, Talmor DS, Thompson BT, Brower RG, Fan E, (2017) Higher PEEP versus Lower PEEP Strategies for Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 14: S297-S303 109. National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF,
Jr., Hite RD, Harabin AL, (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564-2575 110. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C, (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis;doi: 10.1016/S1473-3099(20)30086-4 111. Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA, Repetto CA, Romero CM, Galvez LR, Llanos O, Arellano DH, Neira WR, Diaz GA, Zamorano AJ, Pereira GL, (2013) Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 188: 440-448 112. Albert RK, Hubmayr RD, (2000) The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 161: 1660-1665 113. Nyren S, Radell P, Lindahl SG, Mure M, Petersson J, Larsson SA, Jacobsson H, Sanchez-Crespo A, (2010) Lung ventilation and perfusion in prone and supine postures with reference to anesthetized and mechanically ventilated healthy volunteers. Anesthesiology 112: 682-687 114. Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, Uleryk E, Mancebo J, Pesenti A, Ranieri VM, Fan E, (2017) Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 14: S280-S288 115. Bloomfield R, Noble DW, Sudlow A, (2015) Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev: CD008095 116. Mora-Arteaga JA, Bernal-Ramirez OJ, Rodriguez SJ, (2015) The effects of prone position ventilation in patients with acute respiratory distress syndrome. A systematic review and metaanalysis. Med Intensiva 39: 359-372 117. Lee JM, Bae W, Lee YJ, Cho YJ, (2014) The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med 42: 1252-1262 118. van der Voort PH, Zandstra DF, (2001) Enteral feeding in the critically ill: comparison between the supine and prone positions: a prospective crossover study in mechanically ventilated patients. Crit Care 5: 216-220 119. Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain ML, Montejo Gonzalez JC, Paugam-Burtz C, Poeze M, Preiser JC, Singer P, van Zanten AR, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM, Function EWGoG, (2017) Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 43: 380-398 120. Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, McGee W, McManus C, Meade M, Nix S, Patterson A, Sands MK, Pino R, Tescher A, Arbour R, Rochwerg B, Murray CF, Mehta S, (2016) Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Crit Care Med 44: 2079-2103 121. Griffiths M, Fan E, Baudouin SV, (2019) New UK guidelines for the management of adult patients with ARDS. Thorax 74: 931-933 122. Claesson J, Freundlich M, Gunnarsson I, Laake JH, Moller MH, Vandvik PO, Varpula T, Aasmundstad TA, (2016) Scandinavian clinical practice guideline on fluid and drug therapy in adults with acute respiratory distress syndrome. Acta Anaesthesiol Scand 60: 697-709 123. Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guerin C, Jaber S, Mekontso-Dessap A, Mercat A, Richard JC, Roux D, Vieillard-Baron A, Faure H, (2019) Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care;9: 69 124. Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, Meade MO, (2013) Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta- analysis of randomized controlled trials. Crit Care 17: R43 125. National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ,
Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC, (2019) Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 380: 1997-2008 126. Gebistorf F, Karam O, Wetterslev J, Afshari A, (2016) Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev: CD002787 127. Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G, (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354: 1775-1786 128. Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martinez D, Hernandez M, Tucci M, Borges JB, Lubillo S, Santos A, Araujo JB, Amato MB, Suarez-Sipmann F, Open Lung Approach N, (2016) Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial. Crit Care Med 44: 32-42 129. Xi XM, Jiang L, Zhu B, group RM, (2010) Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl) 123: 3100-3105 130. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I, Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, Romano ER, Regenga MM, Taniguchi LNT, Teixeira C, Pinheiro de Oliveira R, Machado FR, Diaz-Quijano FA, Filho MSA, Maia IS, Caser EB, Filho WO, Borges MC, Martins PA, Matsui M, Ospina-Tascon GA, Giancursi TS, Giraldo-Ramirez ND, Vieira SRR, Assef M, Hasan MS, Szczeklik W, Rios F, Amato MBP, Berwanger O, Ribeiro de Carvalho CR, (2017) Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 318: 1335-1345 131. Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, Keating JL, Pilcher DV, Westbrook AJ, Cooper DJ, Nichol AD, (2011) A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care 15: R133 132. Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, Brickell K, Davies A, Fahey C, Fraser J, McGuinness S, Murray L, Parke R, Paul E, Tuxen D, Vallance S, Young M, Nichol A, (2019) Maximal Recruitment Open Lung Ventilation in Acute Respiratory Distress Syndrome (PHARLAP). A Phase II, Multicenter Randomized Controlled Clinical Trial. Am J Respir Crit Care Med 200: 1363-1372 133. Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y, (2009) Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care 13: R22 134. Goligher EC, Hodgson CL, Adhikari NKJ, Meade MO, Wunsch H, Uleryk E, Gajic O, Amato MPB, Ferguson ND, Rubenfeld GD, Fan E, (2017) Lung Recruitment Maneuvers for Adult Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 14: S304-S311 135. Alshahrani MS, Sindi A, Alshamsi F, Al-Omari A, El Tahan M, Alahmadi B, Zein A, Khatani N, Al- Hameed F, Alamri S, Abdelzaher M, Alghamdi A, Alfousan F, Tash A, Tashkandi W, Alraddadi R, Lewis K, Badawee M, Arabi YM, Fan E, Alhazzani W, (2018) Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care 8: 3 136. Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, Eolia Trial Group R, Ecmonet, (2018) Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 378: 1965-1975 137. Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Juni P, Brodie D, Slutsky AS, Combes A, (2018) Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress
Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 320: 2251-2259 138. Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E, (2019) Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta- analysis. Lancet Respir Med 7: 163-172 139. MacLaren G, Fisher D, Brodie D, (2020) Preparing for the Most Critically Ill Patients With COVID- 19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA;doi: 10.1001/jama.2020.2342 140. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G, (2014) Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 66: 2613-2620 141. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet; DOI: https://doi.org/10.1016/S0140-6736(20)30628-0 142. Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, Dong N, Tong Q, (2020) Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv: 2020.2003.2006.20032342 143. Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, Alexander PE, Fei Y, Vandvik PO, Loeb M, Guyatt GH, (2015) Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med 163: 519-528 144. Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS, (2019) Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2: CD010406 145. Lewis SR, Pritchard MW, Thomas CM, Smith AF, (2019) Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev 7: CD004477 146. Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez- Higueras E, Conesa LA, Martin-Rodriguez C, Diaz-Dominguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Anon JM, Fernandez RL, Gonzalez-Martin JM, dexamethasone in An, (2020) Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 8: 267-276 147. Ranieri VM, Pettila V, Karvonen MK, Jalkanen J, Nightingale P, Brealey D, Mancebo J, Ferrer R, Mercat A, Patroniti N, Quintel M, Vincent JL, Okkonen M, Meziani F, Bellani G, MacCallum N, Creteur J, Kluge S, Artigas-Raventos A, Maksimow M, Piippo I, Elima K, Jalkanen S, Jalkanen M, Bellingan G, Group IS, (2020) Effect of Intravenous Interferon beta-1a on Death and Days Free From Mechanical Ventilation Among Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA;doi: 10.1001/jama.2019.22525 148. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y, (2020) Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med;doi: 10.1001/jamainternmed.2020.0994 149. Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S, Gossack-Keenan K, Alghuroba M, Szczeklik W, Menon K, Alhazzani W, Sevransky J, Vandvik PO, Annane D, Guyatt G, (2018) Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med 46: 1411-1420 150. Lian XJ, Huang DZ, Cao YS, Wei YX, Lian ZZ, Qin TH, He PC, Liu YH, Wang SH, (2019) Reevaluating the Role of Corticosteroids in Septic Shock: An Updated Meta-Analysis of Randomized Controlled Trials. Biomed Res Int 2019: 3175047 151. Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al- Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A,
Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA, Saudi Critical Care Trial G, (2018) Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med 197: 757-767 152. Hui DS, (2018) Systemic Corticosteroid Therapy May Delay Viral Clearance in Patients with Middle East Respiratory Syndrome Coronavirus Infection. Am J Respir Crit Care Med 197: 700-701 153. Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM, (2004) Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol 31: 304-309 154. Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA, Hirshon JM, Ison MG, Johnston BL, Knight SL, McGeer A, Riley LE, Wolfe CR, Alexander PE, Pavia AT, (2019) Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 68: 895-902 155. Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, Shalhoub S, Almotairi A, Al Khatib K, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Al Mekhlafi GA, Al Harthy A, Kharaba A, Ahmadi MA, Sadat M, Mutairi HA, Qasim EA, Jose J, Nasim M, Al-Dawood A, Merson L, Fowler R, Hayden FG, Balkhy HH, Saudi Critical Care Trial G, (2017) Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit Care Med 45: 1683-1695 156. Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR, 3rd, Higgs E, Randolph AG, Smoot BE, Thompson BT, Network NA, (2012) Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 40: 1487-1498 157. Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR, (2010) 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 177: 166-175 158. McCullers JA, (2013) Do specific virus-bacteria pairings drive clinical outcomes of pneumonia? Clin Microbiol Infect 19: 113-118 159. Alfonso J. Rodriguez-Morales, Jaime A. Cardona-Ospina, Estefanía Gutiérrez-Ocampo, Rhuvi Villamizar-Peña, Yeimer Holguin-Rivera, Juan Pablo Escalera-Antezana, Lucia Elena Alvarado- Arnez, D. Katterine Bonilla-Aldana, Carlos Franco-Paredes, Andrés F. Henao-Martinez, Alberto Paniz-Mondolfi, Guillermo J. Lagos-Grisales, Eduardo Ramírez-Vallejo, Jose A. Suárez, Lysien I. Zambrano, Wilmer E. Villamil-Gómez, Graciela J. Balbin-Ramon, Ali A. Rabaan, Harapan Harapan, Kuldeep Dhama, Hiroshi Nishiura, Hiromitsu Kataoka, Tauseef Ahmad, Ranjit Sah, Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis,Travel Medicine and Infectious Disease, 2020, https:// www.sciencedirect.com/science/article/abs/pii/S1477893920300910?via%3Dihub 160. Schulman CI, Namias N, Doherty J, Manning RJ, Li P, Elhaddad A, Lasko D, Amortegui J, Dy CJ, Dlugasch L, Baracco G, Cohn SM, (2005) The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt) 6: 369-375 161. Young P, Saxena M, Bellomo R, Freebairn R, Hammond N, van Haren F, Holliday M, Henderson S, Mackle D, McArthur C, McGuinness S, Myburgh J, Weatherall M, Webb S, Beasley R, Investigators H, Australian, New Zealand Intensive Care Society Clinical Trials G, (2015) Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N Engl J Med 373: 2215-2224 162. Haupt MT, Jastremski MS, Clemmer TP, Metz CA, Goris GB, (1991) Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. The Ibuprofen Study Group. Crit Care Med 19: 1339-1347
163. Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, Higgins SB, Swindell BB, (1997) The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med 336: 912-918 164. Gozzoli V, Schottker P, Suter PM, Ricou B, (2001) Is it worth treating fever in intensive care unit patients? Preliminary results from a randomized trial of the effect of external cooling. Arch Intern Med 161: 121-123 165. Memis D, Karamanlioglu B, Turan A, Koyuncu O, Pamukcu Z, (2004) Effects of lornoxicam on the physiology of severe sepsis. Crit Care 8: R474-482 166. Honarmand H, Abdollahi M, Ahmadi A, Javadi MR, Khoshayand MR, Tabeefar H, Mousavi S, Mahmoudi L, Radfar M, Najafi A, Mojtahedzadeh M, (2012) Randomized trial of the effect of intravenous paracetamol on inflammatory biomarkers and outcome in febrile critically ill adults. Daru 20: 12 167. Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, Dellamonica J, Bouadma L, Cook F, Beji O, Brun-Buisson C, Lemaire F, Brochard L, (2012) Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 185: 1088-1095 168. Niven DJ, Stelfox HT, Leger C, Kubes P, Laupland KB, (2013) Assessment of the safety and feasibility of administering antipyretic therapy in critically ill adults: a pilot randomized clinical trial. J Crit Care 28: 296-302 169. Yang YL, Liu DW, Wang XT, Long Y, Zhou X, Chai WZ, (2013) Body temperature control in patients with refractory septic shock: too much may be harmful. Chin Med J (Engl) 126: 1809-1813 170. Janz DR, Bastarache JA, Rice TW, Bernard GR, Warren MA, Wickersham N, Sills G, Oates JA, Roberts LJ, 2nd, Ware LB, Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Study G, (2015) Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med 43: 534-541 171. Schortgen F, Charles-Nelson A, Bouadma L, Bizouard G, Brochard L, Katsahian S, (2015) Respective impact of lowering body temperature and heart rate on mortality in septic shock: mediation analysis of a randomized trial. Intensive Care Med 41: 1800-1808 172. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L, (2020) Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clinical Infectious Diseases;doi: 10.1093/cid/ciaa199 173. Stiehm ER, (2013) Adverse effects of human immunoglobulin therapy. Transfus Med Rev 27: 171- 178 174. Davey RT, Jr., Fernández-Cruz E, Markowitz N, Pett S, Babiker AG, Wentworth D, Khurana S, Engen N, Gordin F, Jain MK, Kan V, Polizzotto MN, Riska P, Ruxrungtham K, Temesgen Z, Lundgren J, Beigel JH, Lane HC, Neaton JD, Davey RT, Fernández-Cruz E, Markowitz N, Pett S, Babiker AG, Wentworth D, Khurana S, Engen N, Gordin F, Jain MK, Kan V, Polizzotto MN, Riska P, Ruxrungtham K, Temesgen Z, Lundgren J, Beigel JH, Lane HC, Neaton JD, Butts J, Denning E, DuChene A, Krum E, Harrison M, Meger S, Peterson R, Quan K, Shaughnessy M, Thompson G, Vock D, Metcalf J, Dewar R, Rehman T, Natarajan V, McConnell R, Flowers E, Smith K, Hoover M, Coyle EM, Munroe D, Aagaard B, Pearson M, Cursley A, Webb H, Hudson F, Russell C, Sy A, Purvis C, Jackson B, Collaco-Moraes Y, Carey D, Robson R, Sánchez A, Finley E, Conwell D, Losso MH, Gambardella L, Abela C, Lopez P, Alonso H, Touloumi G, Gioukari V, Anagnostou O, Avihingsanon A, Pussadee K, Ubolyam S, Omotosho B, Solórzano C, Petersen T, Vysyaraju K, Rizza SA, Whitaker JA, Nahra R, Baxter J, Coburn P, Gardner EM, Scott JA, Faber L, Pastor E, Makohon L, MacArthur RA, Hillman LM, Farrough MJ, Polenakovik HM, Clark LA, Colon RJ, Kunisaki KM, DeConcini M, Johnson SA, Wolfe CR, Mkumba L, Carbonneau JY, Morris A, Fitzpatrick ME, Kessinger CJ, Salata RA, Arters KA, Tasi CM, Panos RJ, Lach LA, Glesby MJ, Ham KA, Hughes VG, Schooley RT, Crouch D, Muttera L, Novak RM, Bleasdale SC, Zuckerman AE, Manosuthi W,
Thaonyen S, Chiewcharn T, Suwanpimolkul G, Gatechumpol S, Bunpasang S, Angus BJ, Anderson M, Morgan M, Minton J, Gkamaletsou MN, Hambleton J, Price DA, Llewelyn MJ, Sweetman J, Carbone J, Arribas JR, Montejano R, Lobo Beristain JL, Martinez IZ, Barberan J, Hernandez P, Dwyer DE, Kok J, Borges A, Brandt CT, Knudsen LS, Sypsas N, Constantinou C, Markogiannakis A, Zakynthinos S, Katsaounou P, Kalomenidis I, Mykietiuk A, Alzogaray MF, Obed M, Macias LM, Ebensrtejin J, Burgoa P, Nannini E, Lahitte M, Perez-Patrigeon S, Martínez-Orozco JA, Ramírez- Hinojosa JP, Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. The Lancet Respiratory Medicine;7:951-963 175. Beigel JH, Nam HH, Adams PL, Krafft A, Ince WL, El-Kamary SS, Sims AC, (2019) Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference. Antiviral research 167: 45-67 176. Arabi YM, Fowler R, Hayden FG, (2020) Critical care management of adults with community- acquired severe respiratory viral infection. Intensive Care Med 46: 315-328 177. Casadevall A, Pirofski L-a, (2020) The convalescent sera option for containing COVID-19. The Journal of clinical investigation;doi: 10.1172/JCI138003 178. Hung IF, To KK, Lee CK, Lee KL, Yan WW, Chan K, Chan WM, Ngai CW, Law KI, Chow FL, Liu R, Lai KY, Lau CC, Liu SH, Chan KH, Lin CK, Yuen KY, (2013) Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 144: 464-473
|
|||||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2020-11-11; просмотров: 255; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.217.220.114 (0.277 с.) |




 Терапия спасения/ Дополнительная терапия
Терапия спасения/ Дополнительная терапия












 Постановка вопроса
Постановка вопроса
 1
1


 ... о о
... о о