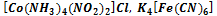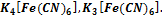Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Chemistry 5B072600, 5B073300 (300 tests)Содержание книги
Поиск на нашем сайте Chemistry 5B072600, 5B073300 (300 tests) 1. The elementary object is …
2. Specify the Avogadro number (NA) А) 6,02·1023 В) 6,02·10-23 С) 6,02·1010 D) 6,02·1015 Е) 6,02·10-10 3. Mathematical expression of the law of equivalents for reaction аА + вВ → сС is: A. n (1/ZAA) = n (1/ZBB) B. m (A) = C (1/ZAA) M (1/ZAA) V (A)/1000 C. T (B/A) = m (A)/V (B) D. n (1/ZAA) = C (1/ZAA) V (A) E. C (1/ZAA) V (A) = C (1/ZBB) V (B) 4. Finish the definition: molar concentration of substance A is …
5. Finish the definition: normal concentration of substance A is …
6. Finish the definition: molar concentration of equivalents of substance А is …
7. Finish the definition: mass concentration of substance A is …
8. Finish the definition: the titer of substance A is …
9. Finish the definition: the mass fraction of substance A is …
10. Finish the definition: the density of solution A is …
Unit of a titer
Unit of molar concentration
Unit of normal concentration
Unit of a mass fraction A. % B. mol/l C. g/ml D. mg / l E. g/l Unit of the number of substance A
Unit of the number of equivalents of substance A A. mol B. g C. there is no unit D. mol/l E. g/mol Unit of an equivalent of substance A
Unit of the molar mass of the substance A
Unit of the molar mass of an equivalent of the substance A
Unit of a number of equivalence of the substance A
Unit of the factor of equivalence
Unit of a density of a solution A
Calculate the normal concentration of the sulfuric acid if its titer is equal 0.02446 g/ml.
Calculate the molar concentration of sodium hydroxide if its titer is equal 0.004020 g/ml.
Calculate the titer of the sulfuric acid, if its normal concentration is 0.1008 mol/l.
Calculate the titer of sodium hydroxide, if its molar concentration is 0.09981 mol/l.
Calculate the molar concentration of the sulfuric acid if its titer is equal 0.004852 g/ml.
Calculate the normal concentration of the sulfuric acid if its titer is equal 0.004852 g/ml
Alkane with octane number 0 A) heptane В) octane С) hexane D) propane Е) pentane
Specify amphoteric oxides А) Al2O3;ZnO В) CuO;Na2O С) ZnO;CO2 D) Na2O;Fe2O3 Е) FeO;P2O5
Specify the acid oxides А) CO2;P2O5 В) CaO;SO3 С) P2O5;Fe2O3 D) Al2O3;K2O Е) ZnO;CrO3
Specify the chloride-ion А) Cl- B) Cl2- С) Cl02 D) Cl4- E) ClO-4
Specify sulfate - ion А) SO2-4 В) SO-4 С) SO3-4 D) HSO2-4 Е) HS- 295. To define coordination numbers of forming complexes in compounds: K A) 2, 6, 6 B) 1, 4, 3 C) 1, 5, 3 D) 0, 3, 3 E) 1, 4, 3
296. To define a charge of forming complex in compound A) +2, +3 B) +1, +2 C) +1, -1 D) +2, +5 E) +1, +6
297. What is the type of reaction H2SO4+ 2NaOH =Na2SO4+ 2Н2O: Chemistry 5B072600, 5B073300 (300 tests) 1. The elementary object is …
2. Specify the Avogadro number (NA) А) 6,02·1023 В) 6,02·10-23 С) 6,02·1010 D) 6,02·1015 Е) 6,02·10-10 3. Mathematical expression of the law of equivalents for reaction аА + вВ → сС is: A. n (1/ZAA) = n (1/ZBB) B. m (A) = C (1/ZAA) M (1/ZAA) V (A)/1000 C. T (B/A) = m (A)/V (B) D. n (1/ZAA) = C (1/ZAA) V (A) E. C (1/ZAA) V (A) = C (1/ZBB) V (B) 4. Finish the definition: molar concentration of substance A is …
5. Finish the definition: normal concentration of substance A is …
6. Finish the definition: molar concentration of equivalents of substance А is …
7. Finish the definition: mass concentration of substance A is …
8. Finish the definition: the titer of substance A is …
9. Finish the definition: the mass fraction of substance A is …
10. Finish the definition: the density of solution A is …
Unit of a titer
Unit of molar concentration
|
||
|
Последнее изменение этой страницы: 2016-12-30; просмотров: 99; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.169 (0.009 с.) |

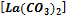 ,
,