Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
What reactions belong to the oxidation-reduction?Содержание книги
Поиск на нашем сайте
2. 3. 4. Fe+S=FeS A) 2, 4 B) 1, 3 C) 2, 3 D) 1, 4 E) 2, 1 299. What is the type of reaction: A) Disproportion B) Neutralization C) Precipitate D) Decomposition E) Exchange 300. Oxidizer: Accepts electrons; Gives electrons; Replaces protons; Reduces enthalpy; Absorbs warmth. 301. Reducer: A) Gives electrons; B) Accepts electrons; C) Replaces protons; D) Reduces enthalpy; E) Absorbs warmth. 302. Oxidizer for the given reaction: A) B) C) D) E)
303. Reducer for the given reaction: 4NH3+3O2=2N2+6H2O A. N-3 B. H+1 C. O0 D. N0 E. O-2
304. To specify the sum of coefficients for reaction: A) 10 B) 8 C) 6 D) 2 E) 4 305. To specify the sum of coefficients for reaction: А)20 Б)12 В)10 Г)14 Д)11 306. To specify the sum of coefficients for reaction: K2S +K2Cr2 А) 26 Б) 20 В) 24 Г) 18 Д)22
307. To specify the sum of coefficients for reaction: A.7 B.8 C.6 D.5 E.3 308. To specify the sum of coefficients for reaction: A) 13 B) 10 C) 9 D) 7 E) 14 309. To specify the sum of coefficients for reaction: A) 19 B) 17 C) 14 D) 21 E) 13 310. To specify the sum of coefficients for reaction: A) 7 B) 6 C) 4 D) 8 E) 5 311. To specify the sum of coefficients for reaction: A) 22 B) 20 C) 18 D) 16 E) 14 312. To specify the sum of coefficients for reaction: A) 10 B) 8 C) 6 D) 4 E) 5 313. To specify the sum of coefficients for reaction: A) 14 B) 12 C) 10 D) 8 E) 6 314. pH of solution calculates according to the formula: A) pH = 14 - pOH B) pH = 14 + pOH C) pH = KgC + 10 D) pH = N/n E) pH = n/N 315. The hydroxyl indicator of environment is equal: A) pH = [H+][OH-] B) рОН = -lg[H+] C) [pОH = - lg[ОH-] D) H2O] = -lg[OH-] E) pOH= -lg[H+][OH-] 316. To specify an alkaline solution: A) [H+] = 10-12 B) [H+] = 10-7 C) [ОH-] = 1031 D) [ОH-] = 10-3 E) [H 2О] = 107 317. To specify a neutral solution: A) H+] = [OH-] B) [H+] > [OH-] C) [H+] < [OH-] D) pH = 12 E) pH = 17
318. Electrolysis is: A) oxidation-reduction process; B) disintegration of a molecule of electrolit to ions; C) process of association of ions; D) movement of electrons around of nucleus; E) process of molecule dissociation.
319. Cathode is an electrode at which there is a process: A) Reduction of cations; B) Oxidation of anions; C) Disintegration of molecules; D) Association of ions; E) Changing of oxidation degree of an element. 320. Anode is an electrode at which there is a process: A) Oxidation of anions; B) Reduction of cations; C) Disintegration of molecules; D) Association of ions; E) Changing of oxidation degree of an element. 321. E lectrolyte of the lead accumulator is the solution of: A) H2SO4 B) H2O C) H3PO4 D) HF E) HCI
322. Inhibitor is: diminish of corrosion corrosion accelerator protector accumulator galvanic cell.
323. Faraday number is equal: A) 96500 B) 73500 C) 760 D) 101 E) 83100 324. Potential of a standard hydrogen electrode is equal: A) 0 B) 1 C) 2 D) 5 E) 9 325. To inert anodes belong: A) graphite, platinum B) zinc, copper C) chrome, manganese D) zinc, chrome E) iron, zinc 326. To active anodes belong: A) copper B) platinum C) neon D) graphite E) plastic 327. At electrolysis of NaCl solution at the anode it is allocated: A) chlorine B) sodium C) oxygen D) hydrogen E) water
|
||
|
Последнее изменение этой страницы: 2016-12-30; просмотров: 100; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.169 (0.008 с.) |

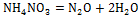 ;
;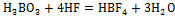 ;
;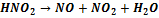 ?
?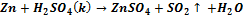
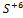 ,
,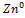 ,
,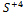 ,
,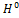 ,
,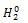
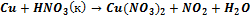
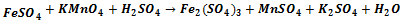
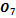 +H2SО4
+H2SО4 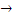 S + K2S04 + Cr2 (SО4
S + K2S04 + Cr2 (SО4 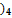 +
+ 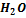
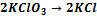 +
+ 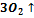
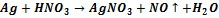
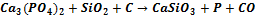
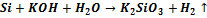
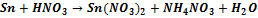
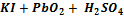 →
→ 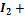
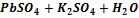
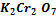 +
+ 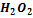 +
+ 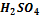 →Cr
→Cr 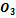 +
+ 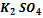 +
+ 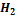 O
O