Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Laboratory work № 7. Determination of the ratio of the specific heat cp/cv for gasesСодержание книги
Похожие статьи вашей тематики
Поиск на нашем сайте
THE AIM: To determine a ratio of the specific heat at constant pressure to specific heat at constant volume INSTRUMENTATION AND APPLIANCES: the device for a ratio of specific heat Cp/Cv determination, manometer.
The description of the device and method of measurements
The device consists of a glass sphere of 12 liters volume, goffered tube, rod, handle of the spring valve, stopper, handle of a rod, water manometer. The sphere can incorporate to an atmosphere with the help of the spring valve. It is possible to increase or to reduce volume of gas in a sphere it is possible at the expense of a stretching or compression goffered tube at lowering or rise of the handle of a rod. The stopper is used for fixation of a rod in the top position. The difference between the pressure of air inside and outside of the sphere is measured by water manometer. If to move a rod downwards up to the stop, and then quickly delay downwards spring valve, the pressure of air inside a sphere becomes equal to atmospheric pressure outside of a sphere. At movement of a rod upwards up to a stop there is action of force on air in a sphere. Thus the external force makes above air some certain work. As a result the internal energy of air in a sphere is increased, and its temperature becomes above room one. The compression of air occurs as adiabatic process. During some time (approximately two minutes) occur reductions of temperature of air in a sphere up to a room temperature. Thus the difference between levels of a liquid in manometer is decreased. It is possible to consider reduction of temperature of air in a sphere as the process at a constant volume. If to pull down handle of the spring valve within 0,5 seconds, the part of air will exhaust for an atmosphere. Thus, air, which has remained in a sphere, does some work on expansion of gas. Thus, the internal energy of air which has stayed in a sphere, decreases, and its temperature becomes lower than a room temperature, and its pressure will be equal to the atmospheric pressure. It is possible to consider expansion of air as adiabatic process. Temperature of air in a sphere will be raised up to a room temperature in two minutes. As a result, the pressure in a sphere will increase, and the difference of levels of a liquid in manometer will increase.
Experimental part
1. Pull down to stopper the handle of a rod. 2. Pull down to stopper the handle of the spring valve till the levels of a liquid in manometer become equal, and release the handle. 3. Lift the handle of a rod and fix by its stopper. 4. In two minutes you have to write down value of a difference of levels of a liquid in manometer in your experimental table - h1. 5. Pull down handle of the spring valve and keep it in this position within 0.5 seconds. 6. In two minutes you have to write down value of a difference of levels of a liquid in manometer into your experimental table - h2. 7. Repeat researches on 1 ÷ 6 two times more. 8. Calculate the experimental value of ratio Cp/Cv by means of the formula
9. Calculate a relative mistake by means of the formula:
10. Determine a confidential interval by means of the formula
11. Calculate magnitude Cp/Cv for air by means of the formula
Compare the ratio Cp /Cv to the magnitude, received in experiment. For two-atomic gas i = 5, for three-atomic gas i = 6.
Control questions
1. List processes occurring in ideal gases. Explain difference between them. 2. Formulate and write down the first law of thermodynamics. 3. Write down the first law of thermodynamics for each of isoprocess. 4. Give definition of a number of degrees of freedom of gas molecules. 5. Write down the formula to calculate the average value of kinetic energy of translation motion of a molecule with use of number of degrees of freedom. 6. What average energy corresponds to one degree of freedom of a molecule of gas? 7. By what formula is the internal energy of ideal gas determined? 8. Give definition of heat capacity of a body and specific heat. 9. Why is specific heat at constant pressure more then specific heat at constant volume? 10. How are Cp and Cv connected to number of degrees of freedom? 11. Write down the formula to calculate the ratio of Cp/Cv with using of number of degrees of freedom.
Author: S.P. Lushchin, the reader, candidate of physical and mathematical sciences. Reviewer: S.V. Loskutov, professor, doctor of physical and mathematical sciences. Approved by the chair of physics. Protocol № 3 from 01.12.2008.
Інструкція З охорони праці № 64
|
||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2016-04-08; просмотров: 473; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.144.227.73 (0.008 с.) |

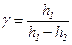 .
.


 You have to take the magnitudes of h1 and h2 from any of the three carried out experiments, and find a confidential interval under the formula for unitary measurement. For a scale manometer an error is equal to 1 mm and error of readings is equal to 0.5 mm.
You have to take the magnitudes of h1 and h2 from any of the three carried out experiments, and find a confidential interval under the formula for unitary measurement. For a scale manometer an error is equal to 1 mm and error of readings is equal to 0.5 mm.