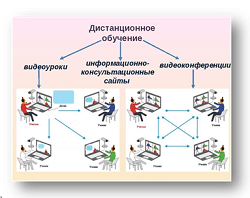
Мы поможем в написании ваших работ!
ЗНАЕТЕ ЛИ ВЫ?
|
The history of investigations of the genetic transformation of animal cells
In 1974 Rudolf Jaenisch created the first genetically modified animal by inserting a DNA virus into an early-stage mouse embryo and showing that the inserted genes were present in every cell.[1] However the mice did not pass the transgene to their offspring. In 1981 the laboratories of Frank Ruddle from Yale, Frank Constantini and Elizabeth Lacy from Oxford, and Ralph Brinster and Richard Palmiter from University of Pennsylvania and University of Washington injected purified DNA into a single-cell mouse embryo and showed transmission of the genetic material to subsequent generations. During the early eighties the technology used to generate genetically modified mice was improved into a tractable and reproducible method.Transformation of animal and plant cells was also investigated with the first transgenic mouse being created by injecting a gene for a rat growth hormone into a mouse embryo in 1982
| | |
38) Use of genes encoding enzymes of nucleotide synthesis as markers (ТК, GFRT, DHPR)
The Escherichia coli dihydrofolate reductase (DHFR) gene has been used as a genetic marker specifying trimethoprim resistance (TmpR). In order to use the DHFR gene as a versatile expression marker, we have constructed three types of plasmids: promoter cloning vector, terminator cloning vector, and the plasmid containing the DHFR gene cassette. In these systems, the selection of recombinant plasmids was carried out just by examining the TmpR phenotype of the transformed cells. Then, levels of the enzymatic activity of DHFR were measured to evaluate the efficiency of promoters and terminators in the fused DNA fragment. An expression plasmid which resulted in the E. coli host cells being able to produce DHFR up to 20% of total cellular proteins was also constructed by changing the promoter and Shine-Dalgarno sequences of the DHFR gene. hypoxanthine-guanine phosphoribosyltransferase - HPRT.For sophisticated gene targeting procedures requiring two sequential selective steps to operate efficiently it is essential that the marker genes used are not prone to position effects. The double replacement gene targeting procedure, to produce mice with subtle gene alterations, is based on the use of hypoxanthine phosphoribosyltransferase (HPRT) minigenes in HPRT-deficient embryonic stem cells. Our standard HPRTminigene, under the control of the mouse phosphoglycerate kinase-1 gene promoter, was stably expressed at five of six target loci examined. At the remaining locus, DNA ligase I (Lig1), expression of this minigene was highly unstable. A different minigene, under the control of the mouse HPRT promoter and embedded in its natural CpG-rich island, overcame this position effect and was stably expressed when targeted to the identical site in the Lig1 locus. The promoter region of the stably expressed minigene remained unmethylated, while the promoter of the unstably expressed minigene rapidly became fully methylated. The difference in the stability of HPRT minigene expression at the same target locus can be explained in the context of the different lengths of their CpG-rich promoter regions with associated transcription factors and a resulting difference in their susceptibility to DNA methylation, rather than by differences in promoter strength. TK. The antibiotic ganciclovir is used to kill cells. Ganciclovir is a "nucleotide analog," meaning it is structurally similar (but not identical) to the building blocks of DNA. It must be phosphorylated before it can be incorporated into DNA in the target cell. Once it is incorporated, it acts like a monkey wrench in the machinery, preventing normal DNA function and thus killing the cell. The enzyme that acts on the ganciclovir is called thymidine kinase (TK). It adds a phosphate on the antibiotic, inactivating the antibiotic. Mammalian TK does not phosphorylate ganciclovir very efficiently, so mammalian cells are not normally killed by it. TK from the Herpes simplex virus (HSV) does phosphorylate it efficiently, and any mammalian cell transformed with an active HSV TK enzyme will be killed. In this system, a plasmid is constructed with the transgene, the HSV TK gene, and a "recombination site," a stretch of DNA that is recognized by the cellular recombinase enzymes that cut and splice DNA. If the trans-gene is integrated into the chromosome at the site of the normal gene, then the HSV TK gene is eliminated by the cellular "recombinase" enzymes, and the cells are not sensitive to ganciclovir. In improperly transformed cells, the recombinase can't remove the HSV TK gene, and so those cells will be killed when exposed to ganciclovir
40. The use of animals as models of human disease
Animal models serving in research may have an existing, inbred or induced disease or injury that is similar to a human condition. These test conditions are often termed as animal models of disease. The use of animal models allows researchers to investigate disease states in ways which would be inaccessible in a human patient, performing procedures on the non-human animal that imply a level of harm that would not be considered ethical to inflict on a human.[ citation needed ]
To serve as a useful model, a modeled disease must be similar in etiology (mechanism of cause) and function to the human equivalent. Animal models are used to learn more about a disease, its diagnosis and its treatment. For instance, behavioral analogues of anxiety or pain in laboratory animals can be used to screen and test new drugs for the treatment of these conditions in humans. A 2000 study found that animal models concorded (coincided on true positives and false negatives) with human toxicity in 71% of cases, with 63% for nonrodents alone and 43% for rodents alone.[5]
Animal models of disease can be spontaneous (naturally occurring in animals), or be induced by physical, chemical or biological means. For example,
- The use of metrazol (pentylenetetrazol) as an animal model of epilepsy[6]
- Immunisation with an auto-antigen to induce an immune response to model autoimmune diseases such as Experimental autoimmune encephalomyelitis[7]
- Occlusion of the middle cerebral artery as an animal model of ischemic stroke[8]
- Injection of blood in the basal ganglia of mice as a model for hemorrhagic stroke[9][10]
- Infecting animals with pathogens to reproduce human infectious diseases
- Injecting animals with agonists or antagonists of various neurotransmitters to reproduce human mental disorders
- Using ionizing radiation to cause tumors
- Implanting animals with tumors to test and develop treatments using ionizing radiation
- Genetically selected (such as in diabetic mice also known as NOD mice)[11]
- Various animal models for screening of drugs for the treatment of glaucoma
- The use of the ovariectomized rat in osteoporosis research
- Use of Plasmodium yoelii as a model of human malaria [12][13][14]
The increase in knowledge of the genomes of non-human primates and other mammals that are genetically close to humans is allowing the production of genetically engineered animal tissues, organs and even animal species which express human diseases, providing a more robust model of human diseases in an animal model.
46. Environmental Biotechnology. Bioremediation and biodegradation
Environmental biotechnology is biotechnology that is applied to and used to study the natural environment. Environmental biotechnology could also imply that one try to harness biological process for commercial uses and exploitation. The International Society for Environmental Biotechnology defines environmental biotechnology as "the development, use and regulation of biological systems for remediation of contaminated environments (land, air, water), and for environment-friendly processes (green manufacturing technologies and sustainable development)".[1]
Environmental biotechnology can simply be described as "the optimal use of nature, in the form of plants, animals, bacteria, fungi and algae, to produce renewable energy, food and nutrients in asynergistic integrated cycle of profit making processes where the waste of each process becomes the feedstock for another process".[2]
Biodegradation is the chemical dissolution of materials by bacteria or other biological means. Although often conflated, biodegradable is distinct in meaning from compostable. While biodegradable simply means to be consumed by microorganisms and return to compounds found in nature, "compostable" makes the specific demand that the object break down in a compost pile. The term is often used in relation to ecology, waste management, biomedicine, and the natural environment (bioremediation) and is now commonly associated with environmentally friendly products that are capable of decomposing back into natural elements. Organic material can be degraded aerobically with oxygen, or anaerobically, without oxygen. Biosurfactant, an extracellular surfactant secreted by microorganisms, enhances the biodegradation process.
Biodegradable matter is generally organic material such as plant and animal matter and other substances originating from living organisms, or artificial materials that are similar enough to plant and animal matter to be put to use by microorganisms. Some microorganisms have a naturally occurring, microbial catabolic diversity to degrade, transform or accumulate a huge range of compounds including hydrocarbons (e.g. oil),polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), pharmaceutical substances, radionuclides, pesticides[1] and metals. Major methodological breakthroughs in microbial biodegradation have enabled detailed genomic, metagenomic, proteomic, bioinformatic and other high-throughput analyses of environmentally relevant microorganisms providing unprecedented insights into key biodegradative pathways and the ability of microorganisms to adapt to changing environmental conditions.[2] Products that contain biodegradable matter and non-biodegradable matter are often marketed as biodegradable.
Bioremediation is the use of micro-organism metabolism to remove pollutants. Technologies can be generally classified as in situ or ex situ. In situ bioremediation involves treating the contaminated material at the site, while ex situ involves the removal of the contaminated material to be treated elsewhere. Some examples of bioremediation related technologies arephytoremediation, bioventing, bioleaching, landfarming, bioreactor, composting, bioaugmentation, rhizofiltration, and biostimulation.
Bioremediation can occur on its own (natural attenuation or intrinsic bioremediation) or can be spurred on via the addition of fertilizers to increase the bioavailability within the medium (biostimulation). Recent advancements have also proven successful via the addition of matched microbe strains to the medium to enhance the resident microbe population's ability to break down contaminants. Microorganisms used to perform the function of bioremediation are known as bioremediators.[1]
Not all contaminants, however, are easily treated by bioremediation using microorganisms. For example, heavy metals such as cadmium and lead are not readily absorbed or captured by microorganisms. The assimilation of metals such as mercury into the food chain may worsen matters. Phytoremediation is useful in these circumstances because natural plants or transgenic plants are able to bioaccumulate these toxins in their above-ground parts, which are then harvested for removal.[2] The heavy metals in the harvested biomass may be further concentrated by incineration or even recycled for industrial use.
The elimination of a wide range of pollutants and wastes from the environment requires increasing our understanding of the relative importance of different pathways and regulatory networks tocarbon flux in particular environments and for particular compounds, and they will certainly accelerate the development of bioremediation technologies and biotransformation processes.[3]
41) Green biotechnology
Green biotechnology makes use of modern technologies such as recombinant-DNA technology to explore the potential of plants to build a sustainable world. In laboratories all over the world such modern technologies are being uses to unravel the basic mechanisms of the behaviour of plants, such as its growth, or its reactions to biotic and abiotic stresses. Reductionistic approaches have been replaced by looking at the plant as a whole. High-throughput technologies and bio-informatics are used to create understanding of the complex interactions between numerous genes, proteins and metabolites. The knowledge generated, can be used in different ways and in different types of applications. It can lead to changes in crop management, but also lead to new directions in plant breeding, and also to the development of genetically engineered crops.
In a world of global warming green biotechnology can contribute in different ways to decrease carbondioxide emissions. I will shortly discuss two approaches:
- Create significant rises in the per hectare yield of crops to prevent further deforestation.
- Improve the potential of plants as a source for bio-energy, such as bio-ethanol or other types of fuel.
Plants for bio-energy
Plants are already used quite a bit for the production of different biofuels, such as biodiesel or bio-ethanol. Traditionally plants have been developed for providing healthy foods, and that is why corn nowadays have very big stalks, and wheat and rice are not very tall. Crops have not been seriously selected as a source of bio-energy. That means that there is still a lot of breeding potential to develop crops that have far better characteristics for bio-energy. One example that can be given is wood. Wood – or to be more precise: the cellulose and hemicellulose in wood – can be converted to bio-ethanol or other types of biofuel, but the conversion today is still inefficiënt. One technological strategy which also involves modern biotechnology is to develop better enzymes to do the conversion. A second strategy is to alter the wood properties to make it more suitable for the conversion. Poplar trees have been made that have less lignin. This is a sort of glue that is responsible for the enzymes not being able to do their work efficiently. Wood produced in a greenhouse has been shown to produce up till 50% more bio-ethanol than conventional wood. Also traditional breeding can help to improve performance, for instance by developing varieties that are better suited for growing in socalled ‘short rotation’. In short rotation you don’t want one dominant stem, but many equally good growing branches. Short rotation is a modern way of growing woody biomass and yields can go up to 30 tons of dry mass per hectare per year, depending on the circumstances.
The way green biotechnology can contribute to reducing carbondioxide emissions may seem somewhat indirect, but it is real and meaningful. Besides working on yield and the suitability for bio-energy there are other approaches, such as developing crops that can still grow and capture carbondioxide under harsh conditions such as drought, salinity or cold. At least one thing is certain: to fight climate change and become more sustainable we literally have to become greener. We have to grow as much plants as possible.
Green biotechnology is biotechnology applied to agricultural processes. An example would be the selection and domestication of plants viamicropropagation. Another example is the designing of transgenic plants to grow under specific environments in the presence (or absence) of chemicals. One hope is that green biotechnology might produce more environmentally friendly solutions than traditional industrial agriculture. An example of this is the engineering of a plant to express a pesticide, thereby ending the need of external application of pesticides. An example of this would be Bt corn.
43 Grey biotechnology Grey biotechnology is bioprocess tech. Like other applications of biotechnology, modern bioprocess technology is an extension of ancient techniques for developing useful products by taking advantage of natural biological activities. When our early ancestors made alcoholic beverages, they used a bioprocess: the combination of yeast cells and nutrients (cereal grains) formed a fermentation system in which the organisms consumed the nutrients for their own growth and produced by-products (alcohol and carbon dioxide gas) that helped to make the beverage. Although more sophisticated, today's bioprocess technology is based on the same principle: combining living matter (whole organisms or enzymes) with nutrients under the conditions necessary to make the desired end product. Bioprocesses have become widely used in several fields of commercial biotechnology, such as production of enzymes (used, for example, in food processing and waste management) and antibiotics. As techniques and instrumentation are refined, bioprocesses may have applications in other areas where chemical processes are now used. Because bioprocesses use living material, they offer several advantages over conventional chemical methods of production: they usually require lower temperature, pressure, and pH (the measure of acidity); they can use renewable resources as raw materials; and greater quantities can be produced with less energy consumption. In most bioprocesses, enzymes are used to catalyze the biochemical reactions of whole microorganisms or their cellular components. The biological catalyst causes the reactions to occur, but is not itself changed. After a series of such reactions (which take place in large vessels called fermenters or fermentation tanks), the initial raw materials are chemically changed to form the desired end product. Although it sounds quite simple, this procedure presents two major challenges. First, the conditions under which the reactions occur must be rigidly maintained. Temperature, pressure, pH, oxygen content, and flow rate are only a few of the variables that must be kept at very specific levels. With the development of automated and computerized equipment, it is becoming much easier to accurately monitor reaction conditions and thus increase production efficiency. Second, the reactions result in the formation of many unwanted by-products. The presence of contaminating waste material often poses a two-fold problem: how to recover (or separate) the end product in a way that leaves as little residue as possible in the catalytic system (since enzymatic catalysts remain unchanged as they drive reactions, they can be used over and over again); and how to isolate the desired product in pure form.
44) White biotechnology
White biotechnology, or industrial biotechnology as it is also known, refers to the use of living cells and their enzymes to create industrial products that are more easily degradable, require less energy, create less waste during production and sometimes perform better than products created using traditional chemical processes.
White biotechnology uses micro-organisms and enzymes, for example, to convert organic material (from agriculture or household wastes) into fuels for cars or enzyme-based detergents for washing at lower temperatures. New methods of production and new products are being developed in many industrial sectors, such as chemistry, food and feed, paper and pulp, textiles and energy. These applications can impact the economic and social parameters of the economy, making sustainable development a reality in the years to come.
As a result of their potential in the production of high quality products, white biotechnology is an economically very interesting field of business. Biotechnologically produced products are used in human and animal nutrition as well as in the production of agricultural and pharmaceutical products.
In white biotechnology, a distinction is made between fermentative processes and enzymatic processes. In fermentation, the metabolic functions of living microorganisms are utilized. The organisms are "fed" with a raw material, and the finished product is obtained. In biocatalysis a single enzyme is used to catalyze only a specific reaction step. The enzymes employed generally originate from microorganisms.
Biocatalysis
Biocatalysis is an important subspecialty of white biotechnology. They are used primarily to produce chiral intermediates required for the production of medicines and crop protectants.
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds.
Enzymes which are capable of catalyzing reactions which have numerous advantages:
- substrate specificity
-srictly enantioselective and regiospecific
- active under mild conditions of pH and temperature
- do not require the utilization of solvents
As a result, biocatalysis reduces environmental costs of classical synthetic chemistry and participates in the development of “sustainable” chemistry which is becoming more and more important in the politics of development of industrial activities. The synthesis of chemical products thanks to biotechnology processes which use enzymes is called “white biotechnology” and is used by an increasing number of industrialists for the production of organic compounds, products for use in agriculture, pharmaceutical products, food additives and bioplastics.
Fermentation
Fermentation is a metabolic process in which an organism converts a carbohydrate, such as starch or a sugar, into an alcohol or an acid. For example, yeast perform fermentation to obtain energy by converting sugar into alcohol. Bacteria perform fermentation, converting carbohydrates into lactic acid. Fermentation is a process used to produce wine, beer, yogurt and other products.
45) Microorganisms used in fermentation
The diversity of fermentation products produced by the microorganisms is attributed to the rich diversity of microorganisms which have a diverse metabolisms that can yield various types of fermentation products WHY MICROORGANISMS ARE THE BEST CHOICE FOR FERMENTATION? The success of using microorganisms for fermentation lies in their very microscopic and metabolic characteristics. It is good being small!
1 High surface area to volume ratio
Microorganisms are very very tiny creatures. Taking an example of a rod bacterium (imagine a brick!), we can see that it has six free surfaces that surrounds the bacterium. These six free surfaces interfaced with the surrounding environment from where they obtained their nutrients or to where they throw away their metabolic waste products.
With such a high number of free surface areas in a tiny volume of cell, it confers upon the bacterial cell a very high surface area to volume ratio. This very high surface area to volume ratio allows maximum or optimum surfaces for diffusions or molecular exchanges to occur between the microbial cell and the environment. No matter where the molecules are, they are easily accessible for diffusion into the microbial cell.
2 Mode of nutrients transportation
The nutrients which diffuse into the microbial can either use simple diffusion process which is powered by the differences in the concentration gradients between the environment and within the cell. For very small nutrient molecules, most would diffuse by the mechanism of passive diffusion. Larger and complex molecules use active or group transport which requires expansion or utilization of energy Microbes easily reproduce asexually! There is no real need to have a opposite partner cells, get married and reproduce. They will just as easily split their cells into two daughter cells which will later grow into larger cells and repeat the cycle...
3 Genetic adaptability
Microorganisms generally show the ability to adapt to new environment. They can get easily adapted to living under different environmental conditions and also adapting to new sources of carbon or substrate. This ability is the result of various genetic adaptation which selects "successful" strains through mutation and genetic recombination. Some of the bacteria are even equipped with plasmids which can synthesize new enzymes that help the microorganisms exploit the new environment. The very short generation times and the high population generation will aid the selection and recombination process. 4 Metabolic diversity
The unique thing about microbes are their metabolic diversity shown by various members of the microorganisms. They have the ability to use different energy sources and to use different types terminal electron acceptors
Their ability to use different substrates is also correlated with the microbes ability to produce a diversity of fermentation products
47)The methods of experimental Haploid technology The most commonly used method to create haploid plants in a method of in vitro androgenesis - formation of haploid plants from anther cells cporogennoy usually in the phase of vacuolated microspores. However, this process is depends on several interrelated factors, each of which has an influence on the morphogenetic processes when cultured anthers isolated microspores in vitro. For some crops showed that embryogenesis depends on genetic, physiological reasons bud stage of development, and microspores, and environmental factors such as composition of the nutrient medium (mineral and hormonal composition), and culturing conditions (liquid, solid nutrient medium and light temperature regimes) [2,3,9]. The results showed that still embryogenesis in anther culture in vitro occurs spontaneously and has a low frequency output of haploid plants (1 - 4%), and the proposed technology is difficult and poorly reproducible studied at each stage androgenesis [1, 5, 7, 8]. Therefore, the development and improvement of existing methodological approaches, it is necessary to carry out, given the genotypic characteristics of the samples.
The development of these structures occur in two ways: 1) formation of embryos, 2) the formation of elongated cells and their subsequent destruction. Visually it was observed that the formation of embryos occurred only if the structure to fall medium after anther rupture. If the structures remained within the anther, it was observed the formation of elongated cells, which was similar to the process of pollen germination in vivo. One can assume that the lowest frequency of embryogenesis in the culture isolated anther associated with low cell yield structures medium.
49 Somatic hybridisation in vitro. Development of hybrid plants through the fusion of somatic protoplasts of two different plant species/varieties is called somatic hybridization. Somatic hybridization technique: 1. isolation of protoplast from suitable plants 2. Fusion of the protoplasts of desired species/varieties. 3. Identification and Selection of somatic hybrid cells. 4. Culture of the hybrid cells. 5. Regeneration of hybrid plants. Isolation of Protoplast (Separartion of protoplasts from plant tissue) 1. Mechanical Method 2. Enzymatic Method. Protoplast Fusion( Fusion of protoplasts of two different genomes)1. Spontaneous Fusion Protoplast fuse spontaneously during isolation process mainly due to physical contact - Intraspecific produce homokaryones. 2. Induced Fusion. Chemo fusion- fusion induced by chemicals.Types of fusogens – PEG NaNo3 High pH/Ca 2+ ions Polyvinyl alcohal Electrofusion- Fusion induced by electrical stimulation - Pearl chain of protoplasts is formed by low strength electric field (10kv m-1). Fusion of protoplasts of pearl chain is induced by the application of high strength electric field (100kv m-1) for few microseco. High voltage pulse induce a reversible breakdown of plasma membrane at the sit of cell contact, leading to fusion and consequently membrane reorganization. Simple, quicker and more efficient than chemical induced fusion. Cell Wall Regeneration - May be complete in two to several days, Although protoplast in culture generally start regenerating a cell wall within a few hours after isolation, Protoplast lost their characteristic spherical shape once the wall formation is complete, Regeration of cell wall can be demonstrated using Calcalfluor White ST fluoresecent Stain (USA) or Tinapol solution (UK). Hybrid identification- Based on difference between the parental cells and hybrid cell with respect to Pigmentation Cytoplasmic markers Fluorochromes like FITC (fluoroscein isothiocyanate) and RITC (Rhodamine isothiocyanate) are used for labelling of hybrid cellsPresence of chloroplast Nuclear staining, etc. Regeneration of hybrid plants: Plants are induced to regenerate from hybrid calli. These hybrid plants must be at least partially fertile, in addition to having some useful property, to be of any use in breeding schemes. Advantages of somatic hybridization. Production of novel interspecific and intergenic hybrid. Pomato (Hybrid of potato and tomato). Production of fertile diploids and polypoids from sexually sterile haploids, triploids and aneuploids. Transfer gene for disease resistance, abiotic stress resistance, herbicide resistance and many other quality characters. Production of heterozygous lines in the single species which cannot be propagated by vegetative means. Studies on the fate of plasma genes. Production of unique hybrids of nucleus and cytoplasm. Limitations of Somatic hybridization. Poor regeneration of hybrid plants. Non-viability of fused products. Not successful in all plants. Production of unfavorable hybrids. Lack of an efficient method for selection of hybrids. No confirmation of expression of particular trait in somatic hybrids
50) Apply cell technology for biosynthesis of secondary metabolites
Secondary metabolites are substances produced chiefly by micro-organisms and plants. They exhibit a wide range of biological activities and include antibiotics, such as the pencillins, and other medicinals, such as morphine.
Secondary metabolites are organic compounds that are not directly involved in the normal growth, development, or reproduction of an organism. Unlike primary metabolites, absence of secondary metabolites does not result in immediate death, but rather in long-term impairment of the organism's survivability, fecundity, or aesthetics, or perhaps in no significant change at all. Secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavorings, and recreational drugs.Examples for secondary metabolites includes plant chemical defenses against herbivory and antibiotics. The phylogenetic distribution of secondary metabolites is often limited to a narrow set of taxa.
Groups of secondary metabolites: polyketides, terpenes and steroids, shikimate metabolites, alkaloids and microbial metabolites containing nitrogen.
Alkaloids are produced by a large variety of organisms, including bacteria, fungi, plants, and animals, and are part of the group of natural products (also called secondary metabolites). Many alkaloids can be purified from crude extracts by acid-base extraction. Many alkaloids are toxic to other organisms. They often have pharmacological effects and are used as medications, as recreational drugs, or in entheogenic rituals.
Polyketides are secondary metabolites from bacteria, fungi, plants, and animals. Polyketides are usually biosynthesized through the decarboxylative condensation of malonyl-CoA derived extender units in a similar process to fatty acid synthesis (a Claisen condensation). [1] The polyketide chains produced by a minimal polyketide synthase are often further derivitized and modified into bioactive natural products.
The terpenoids, sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in functional groups but also in their basic carbon skeletons. These lipids can be found in all classes of living things, and are the largest group of natural products.
"microbial diversity" rests fundamentally on some aspect procaryotic metabolism, especially with regards to energy-generating metabolism and synthesis of secondary metabolites. Microbial diversity translates to metabolic diversity. The procaryotes, as a group, conduct all the same types of basic metabolism as eucaryotes, but, in addition, there are several types of energy-generating metabolism among the procaryotes that are non existent in eucaryotic cells or organisms. These include
Unique fermentation pathways that produce a wide array of end products
Anaerobic respiration: respiration that uses substances other than O2 as a final electron acceptor
Lithotrophy: use of inorganic substances as sources of energy
Photoheterotrophy: use of organic compounds as a carbon source during bacterial photosynthesis
Anoxygenic photosynthesis: uses special chlorophylls and occurs in the absence of O2
Methanogenesis: an ancient type of archaean metabolism that uses H2 as an energy source and produces methane
Light-driven nonphotosynthetic energy production: unique archaean metabolism that converts light energy into chemical energy; occurs in the archaea (extreme halophiles)
Unique mechanisms for autotrophic CO2 fixation, including primary production on anaerobic habitats.
51) Two methods of plant microclonal propagations
At present, developed a number of different ways biotechnology micropropagation. They are based on four basic approaches:
1) activation of plant meristem (apex escape, axillary and dormant buds shoot);
2) the formation of adventitious buds of tissue explants;
3) the induction of somatic embryogenesis;
4) differentiation of adventitious buds in the primary and transplantable callus tissue.
The main method of micropropagation of plants is the activation of axillary meristems. This method is based on the removal of apical dominance, which can be achieved in two ways:
1. Produce shoots of normal proportions, followed by their separation, which are used as secondary explants to repeat the cycle of reproduction.
2. Introduction to the medium of substances with cytokinin activity, which leads to the formation of shoots with relatively short internodes, and axillary buds give rise to new shoots. The explants on such media take the form of small bundles of shoots, each of which can be reclaimed. In theory, the ability of this method can be up to 15000000000 or kidney shoots in one year from one explants. It is believed that this method has a minimal degree of risk involved in making non-uniform offspring and frequency of mutant plants do not exceed the frequency of occurrence of the conventional breeding.
The second method - is the induction of the formation of adventitious buds directly tissue explants. It is based on the ability of isolated parts of plants under favorable conditions, the culture medium to recover the missing organs and regenerate whole plants (with the exception of the root organogenesis). Almost all the organs and tissues of plants can form adventitious buds. This process usually takes place in nutrient media containing only cytokinins or auxins in combination with a 10:1 or 100:1. Since auxin in this case, the most commonly used PKI or NOC.
In some instances, an effective method of propagating plants in vitro somatic embryogenesis may be - is the formation zarodkopodibnih structures (embryos) from the somatic cells under conditions in vitro, which when transferred to the appropriate culture medium can develop into a whole plant. Somatic embryogenesis demonstrates totipotency of plant cells.
Embryos formed from the single cells are located primarily on the surface of the callus. They are distinguished by a dense cytoplasm, relatively large nucleus with enlarged nucleoli, contain small vacuoles. It is metabolically active cells, rich in proteins and RNA.
Embryoid formation in tissue culture in two phases. In the first step explants dediferentsiyuyutsya cells by adding to the medium Auxin typically 2,4-D, and converted into embryonic. In the next step of these cells developed embryoids. This occurs with a decrease in the concentration of auxin or even its complete exclusion from the culture medium.
Embryogenesis occurs more readily in young cultures. With the prolongation of cultivation embriogenna cell activity weakened. So carrot embryos begin to form within 4-6 weeks after receipt of the callus culture of the optimal age for induction of somatic embryogenesis - 1520 30 weeks 40-week culture often loses embriogenny potential.
The fourth method of micropropagation - differentiation of adventitious buds in the primary and transplantable callus tissue. This method is not used for planting meterial in vitro. This is due to the fact that the long-term cultivation of callus cells observed changes in ploidy, structural chromosome rearrangements, and accumulation of gene mutations and reduction or loss of morphogenic potential. Therefore, this method of reproduction mikroklonoalnogo should be used only for those plants for which is inherent in the genetic stability of callus tissue and the variability between plants regenerated does not exceed the level of natural variability. These plants include tomatoes, asparagus, some tree species. Through callus cultures were also reproduced sugar beets, corn, rice, wheat, sunflower, flax, potatoes, cucumber.
|
|
|