Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
B. Rutherford's nuclear modelСодержание книги
Поиск на нашем сайте
Rutherford overturned Thomson's model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, massive nucleus. Five years earlier Rutherford had noticed that alpha particles, beamed through a hole onto a photographic plate, would make a sharp-edged picture, while alpha particles beamed through a sheet of mica only 20 micrometres (or about 0.002 centimetre) thick would make an impression with blurry edges. For some particles, the blurring corresponded to a two-degree deflection. Remembering those results, Rutherford had his postdoctoral fellow, Hans Geiger, and an undergraduate student, Ernest Marsden, refine the experiment. The young physicists beamed alpha particles through gold foil and detected them as flashes of light or scintillations on a screen. The gold foil was only 0.00004 centimetre thick. Most of the alpha particles went straight through the foil, but some were deflected by the foil and hit a spot on a screen placed off to one side. Geiger and Marsden found that about one in 20,000 alpha particles had been deflected 45° or more. Rutherford asked why so many alpha particles passed through the gold foil while a few were deflected so greatly. “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper, and it came back to hit you,” Rutherford said later. “On consideration, I realized that this scattering backwards must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive centre carrying a charge.”
Many physicists distrusted Rutherford's nuclear model because it was difficult to reconcile with the chemical behaviour of atoms. The model suggested that the charge on the nucleus was the most important characteristic of the atom, determining its structure. On the other hand, Mendeleyev's periodic table of the elements had been organized according to the atomic masses of the elements, implying that the mass was responsible for the structure and chemical behaviour of atoms.
C. Moseley’s model
Henry Gwyn Jeffreys Moseley, a young English physicist killed in World War I, confirmed that the positive charge on the nucleus revealed more about the fundamental structure of the atom than Mendeleyev's atomic mass. Moseley studied the spectral lines emitted by heavy elements in the X-ray region of the electromagnetic spectrum. He built on the work done by several other British physicists—Charles Glover Barkla, who had studied X-rays produced by the impact of electrons on metal plates, and Sir William Bragg and his son Lawrence, who had developed a precise method of using crystals to reflect X-rays and measure their wavelength by diffraction. Moseley used a crystal of potassium ferrocyanide as a diffraction grating to examine the spectra of X-rays produced by different metals. He arranged his crystal so that he could control and vary the angle between the crystal face and the X-ray beam. The X-rays from each element were reflected at a unique set of angles. By measuring the angle, Moseley was able to obtain the wavelength of the X-ray hitting the crystal. Moseley found that the X-rays radiated by each element have a characteristic frequency that differs according to a regular pattern. The difference in frequency is not governed by Mendeleyev's change in mass, however, but rather by the change in charge on the nucleus. He called this the atomic number. In his first experiments, conducted in 1913, Moseley used the K-series of X-rays (X-radiation associated with the K-energy state of an atom) and studied the elements up to zinc. The following year he extended his work up to gold in the periodic table, using the L-series of X –rays (X-radiation associated with the L-atomic-energy state). Moseley was conducting his research at the same time that the Danish theorist Niels (phys.) Bohr was developing his quantum shell model of the atom. The two conferred and shared data as their work progressed and Moseley framed his equation in terms of Bohr's theory. Moseley presented formulas for the X-ray frequencies that were closely related to Bohr's formulas for the spectral lines in a hydrogen atom. Moseley showed that the frequency of a line in the X-ray spectrum is proportional to the square of the charge on the nucleus. The constant of proportionality depends on whether the X-ray is in the K- or L-series. This is the same relationship that Bohr used in his formula applied to the Lyman and Balmer series of spectral lines. The regularity of the differences in X-ray frequencies allowed Moseley to order the elements by atomic number from aluminum to gold. He observed that, in some cases, the order by atomic weights was incorrect. For example, cobalt has a larger atomic mass than nickel, but Moseley found that it has atomic number 27, while nickel has 28. When Mendeleyev constructed the periodic table, he based his system on the atomic masses of the elements and had to put cobalt and nickel out of order to make the chemical properties fit better. In a few places where Moseley found more than one integer between elements, he predicted correctly that a new element would be discovered. Because there is just one element for each atomic number, scientists could be confident for the first time of the completeness of the periodic table; no unexpected new elements would be discovered.
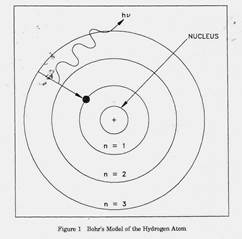
D. Bohr Model of the Atom The British physicist Ernest Rutherford postulated that the positive charge in an atom is concentrated in a small region called a nucleus at the center of the atom with electrons existing in orbits around it. Niels Bohr, coupling Rutherford's postulation with the quantum theory introduced by Max Planck, proposed that the atom consists of a dense nucleus of protons surrounded by electrons traveling in discrete orbits at fixed distances from the nucleus. An electron in one of these orbits or shells has a specific or discrete quantity of energy (quantum). When an electron moves from one allowed orbit to another allowed orbit, the energy difference between the two states is emitted or absorbed in the form of a single quantum of radiant energy called a photon. Figure 1 is Bohr's model of the hydrogen atom showing an electron as having just dropped from the third shell to the first shell with the emission of a photon that has an energy = hv. (h = Planck's constant = 6.63 x 10-34 J-s and v = frequency of the photon.) Bohr's theory was the first to successfully account for the discrete energy levels of this radiation as measured in the laboratory. Although Bohr's atomic model is designed specifically to explain the hydrogen atom, his theories apply generally to the structure of all atoms.
Properties of the three subatomic particles are listed in the Table below. TABLE
|
|||||||||||||||||||||
|
Последнее изменение этой страницы: 2016-08-01; просмотров: 422; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.41 (0.007 с.) |