Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
If you have ticked most of these statements, engineering is the right course of study for you.Содержание книги
Поиск на нашем сайте
CONTENTS Section 1 ATOMIC NATURE OF MATTER Section 2 NUCLIDES Section 3 MASS DEFECT Section 4 BINDING ENERGY Section 5 MODES OF RADIOACTIVE DECAY Section 6 RADIOACTIVITY Section 7 NEUTRON INTERACTIONS Appendix 1 Mathematical Signs, Symbols and Abbreviations Appendix 2 Greek Alphabet Appendix 3 Units and Dimensions Appendix 4 Report and Presentation
REFERENCES
SECTION 1 ATOMIC NATURE OF MATTER LEAD-IN Study this list of points to consider when deciding whether to study engineering. Tick [√] the statements which refer to you. Then ask your partner which statements refer to him or her. 1 You enjoy practical projects - creating and investigating things. 2 You like finding out how things work. 3 You are interested in improving the environment. 4 You like helping people. 5 You enjoy solving problems. 6 You enjoy organizing activities. 7 You enjoy science programmes on TV or on the radio.
If you have ticked most of these statements, engineering is the right course of study for you.
Read the following text and do the tasks below (1-3): In 1661 the English chemist Robert Boyle published the modern criterion for an element. He defined an element to be a basic substance that cannot be broken down into any simpler substance after it is isolated from a compound, but can be combined with other elements to form compounds. To date, 105 different elements have been confirmed to exist, and researchers claim to have discovered three additional elements. Of the 105 confirmed elements, 90 exist in nature and 15 are man-made. Another basic concept of matter that the Greeks debated was whether matter was continuous or.discrete. That is, whether matter could be continuously divided and subdivided into ever smaller particles or whether eventually an indivisible particle would be encountered. Democritus in about 450 B.C. argued that substances were „ultimately composed of small, indivisible particles that he labeled atoms. He further suggested that different substances were composed of different atoms or combinations of atoms, and that one substance could be converted into another by rearranging the atoms. It was impossible to conclusively prove or disprove this proposal for more than 2000 years. The modern proof for the atomic nature of matter was first proposed by the English chemist John Dalton in 1803. Dalton stated that each chemical element possesses a particular kind of atom, and any quantity of the element is made up of identical atoms of this kind. What distinguishes one element from another element is the kind of atom of which it consists, and the basic physical difference between kinds of atoms is their weight.
Complete the following sentences using the information from the text and your knowledge. 1. The English chemist Robert Boyle … 2. The Greeks debated … 3. Democritus argued … 4. The English scientist John Dalton proposed …
Work in pairs. Decide whether the statements below (1-3) are true or false. Correct the false sentences. Share your ideas with other students in your group. 1. Robert Boyle argued that an element can be broken down into any simpler substance after it is isolated from a compound. 2. It was impossible to finally prove or disprove the Democritus suggestion that different substances were composed of different atoms or combinations of atoms, and that one substance could be converted into another by rearranging the atoms. 3. Robert Boyle was the first to prove the atomic nature of matter.
3. Using information from the text say a few words about: 1. the modern criterion for an element published by Robert Boyle 2. continuous or.discrete matter 3. Democritus proposal that was impossible to conclusively prove or disprove this proposal for more than 2000 years 4. the modern proof for the atomic nature of matter proposed by John Dalton in 1803 READING TEXT 1 Before reading the text below complete the sentences 1-3. 1. Subatomic particles are … 2. Gravity, electromagnetism, strong nuclear force and weak nuclear force are … 3. A gauge boson is …
You are going to read a text about subatomic particle theory. Six phrases have been removed from the text. Choose from the sentence A – G the one which fits each gap (1 – 6). There is one extra phrase, which you don’t need to use. A which governs the aggregation of matter B that emerges when a neutron changes by beta decay into a proton C that is independent of charge D which transmits the electromagnetic force between electrically charged objects E that provide this mortar are associated with four basic forces F which acts only between quarks G which incorporates all four fundamental forces Work in pairs or groups. Read the following definitions and decide what they mean. 1. either of the subatomic particles, the proton and the neutron, constituting atomic nuclei. Protons (positively charged) and neutrons (uncharged) behave identically under the influence of the short-range nuclear force, both in the way they are bound in nuclei and in the way they are scattered by each other 2. type of fundamental particle with no electric charge, little or no mass, and one-half unit of spin. Belong to the family of particles called leptons, which are not subject to the strong nuclear force. There are three types, each associated with a charged lepton—i.e., the electron, muon, and tau 3. any member of a group of subatomic particles having odd half-integral angular momentum (spin 1/2, 3/2); named for the Fermi-Dirac statistics that describe its behaviour. Include particles in the class of leptons (e.g., electrons, muons), baryons (e.g., neutrons, protons, lambda particles), and nuclei of odd mass number (e.g., tritium, helium-3, uranium-233) 4. a stable subatomic particle that has a unit-positive charge and a mass of 1.6726231 × 10−27 kg, which is 1,836 times the mass of an electron 5. also called elementary particle any of various self-contained units of matter or energy. More than 200 have been detected, and each appears to have an antiparticle, an antimatter counterpart with the identical mass but opposite electric charge, magnetic moment, or spin 6. lightest stable subatomic particle known. It carries a negative charge which is considered the basic charge of electricity 7. one of the constituent particles of every atomic nucleus except ordinary hydrogen; it has no electrical charge and its mass is nearly 1,840 times that of the electron 8. any member of a class of fermions that respond only to electromagnetic, weak, and gravitational forces and do not take part in strong interactions 9. subatomic particle with integral spin (i.e., angular momentum in quantum-mechanical units of 0, 1, etc.) that is governed by the Bose-Einstein statistics (q.v.). Include mesons (e.g., pions and kaons), nuclei of even mass number (e.g., helium-4), and the particles required to embody the fields of quantum field theory (e.g., photons and gluons) 10. any of a group of subatomic particles believed to be among the fundamental constituents of matter. Constitute all hadrons (baryons and mesons)—i.e., all particles that interact by means of the strong force; the force that binds the components of the nucleus Make a list of collocations with the words below and use them in sentences of your own. Example: motion → molecular motion, motion of the fluid → Molecular motion is induced by the heat.
appearance particle environment experience scale matter force reaction field structure behaviour
Make up a report on the topics below. 1. «Robert Boyle (or other famous scientist) and his Discoveries» 2. «Subatomic Particles» 3. «Nuclear Forces»
(See appendix 4)
TEXT 2
2.1. Work in pairs or groups. Before reading the text below, answer the question: What do you know about: - Thomson's model of atomic structure, - Rutherford's nuclear model, - Moseley’s model, - Bohr model of the atom
Now read the text and check your guesses. Models of Atomic Structure C. Moseley’s model
Henry Gwyn Jeffreys Moseley, a young English physicist killed in World War I, confirmed that the positive charge on the nucleus revealed more about the fundamental structure of the atom than Mendeleyev's atomic mass. Moseley studied the spectral lines emitted by heavy elements in the X-ray region of the electromagnetic spectrum. He built on the work done by several other British physicists—Charles Glover Barkla, who had studied X-rays produced by the impact of electrons on metal plates, and Sir William Bragg and his son Lawrence, who had developed a precise method of using crystals to reflect X-rays and measure their wavelength by diffraction. Moseley used a crystal of potassium ferrocyanide as a diffraction grating to examine the spectra of X-rays produced by different metals. He arranged his crystal so that he could control and vary the angle between the crystal face and the X-ray beam. The X-rays from each element were reflected at a unique set of angles. By measuring the angle, Moseley was able to obtain the wavelength of the X-ray hitting the crystal. Moseley found that the X-rays radiated by each element have a characteristic frequency that differs according to a regular pattern. The difference in frequency is not governed by Mendeleyev's change in mass, however, but rather by the change in charge on the nucleus. He called this the atomic number. In his first experiments, conducted in 1913, Moseley used the K-series of X-rays (X-radiation associated with the K-energy state of an atom) and studied the elements up to zinc. The following year he extended his work up to gold in the periodic table, using the L-series of X –rays (X-radiation associated with the L-atomic-energy state). Moseley was conducting his research at the same time that the Danish theorist Niels (phys.) Bohr was developing his quantum shell model of the atom. The two conferred and shared data as their work progressed and Moseley framed his equation in terms of Bohr's theory. Moseley presented formulas for the X-ray frequencies that were closely related to Bohr's formulas for the spectral lines in a hydrogen atom. Moseley showed that the frequency of a line in the X-ray spectrum is proportional to the square of the charge on the nucleus. The constant of proportionality depends on whether the X-ray is in the K- or L-series. This is the same relationship that Bohr used in his formula applied to the Lyman and Balmer series of spectral lines. The regularity of the differences in X-ray frequencies allowed Moseley to order the elements by atomic number from aluminum to gold. He observed that, in some cases, the order by atomic weights was incorrect. For example, cobalt has a larger atomic mass than nickel, but Moseley found that it has atomic number 27, while nickel has 28. When Mendeleyev constructed the periodic table, he based his system on the atomic masses of the elements and had to put cobalt and nickel out of order to make the chemical properties fit better. In a few places where Moseley found more than one integer between elements, he predicted correctly that a new element would be discovered. Because there is just one element for each atomic number, scientists could be confident for the first time of the completeness of the periodic table; no unexpected new elements would be discovered.
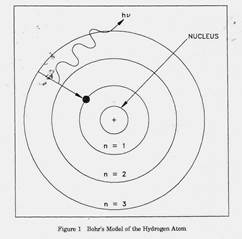
D. Bohr Model of the Atom The British physicist Ernest Rutherford postulated that the positive charge in an atom is concentrated in a small region called a nucleus at the center of the atom with electrons existing in orbits around it. Niels Bohr, coupling Rutherford's postulation with the quantum theory introduced by Max Planck, proposed that the atom consists of a dense nucleus of protons surrounded by electrons traveling in discrete orbits at fixed distances from the nucleus. An electron in one of these orbits or shells has a specific or discrete quantity of energy (quantum). When an electron moves from one allowed orbit to another allowed orbit, the energy difference between the two states is emitted or absorbed in the form of a single quantum of radiant energy called a photon. Figure 1 is Bohr's model of the hydrogen atom showing an electron as having just dropped from the third shell to the first shell with the emission of a photon that has an energy = hv. (h = Planck's constant = 6.63 x 10-34 J-s and v = frequency of the photon.) Bohr's theory was the first to successfully account for the discrete energy levels of this radiation as measured in the laboratory. Although Bohr's atomic model is designed specifically to explain the hydrogen atom, his theories apply generally to the structure of all atoms.
Properties of the three subatomic particles are listed in the Table below. TABLE
Persuasion Ø Don’t you think Ø After all Ø What you don’t seem to understand is that Ø I’m awfully sorry to ask you… but Ø If you do it … I’ll Ø I don’t, but Ø I’ll tell you what (the way) Ø Look Ø Why don’t we Ø I know you can do it Ø It’s crucial for you Ø It’s important for you Ø It’s necessary for you
Going to persuasions Ø (Well) I guess so Ø All right Ø May be you’re right Ø Oh, if you insist Ø Look – I’ll tell you what Ø We’ll see
Making suggestions Ø I wonder/ was wondering how to attend Ø What do you say Ø May be you could Ø That’s a good idea, but Ø That’s might be OK, but Ø That’s true, but Ø I have an idea Ø I think it might be a good idea,but TEXT 3
Isotopes
Isotopesare nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Most elements have a few stable isotopes and several unstable, radioactive isotopes. For example, oxygen has three stable isotopes that can be found in nature (oxygen-16, oxygen-17, and oxygen-18) and eight radioactive isotopes. Another example is hydrogen, which has two stable -isotopes (hydrogen-1 and hydrogen-2) and a single radioactive isotope (hydrogen-3). The isotopes of hydrogen are unique in that they are each commonly referred to by a unique name instead of the common chemical element name. Hydrogen-1 is almost always referred to as hydrogen, but the term protium is infrequently used also. Hydrogen-2 is commonly called deuterium and symbolized 21 D. Hydrogen-3 is commonly called tritium and symbolized 31 T. This text will normally use the symbology 21 H and 31 H for deuterium and tritium, respectively. Evidence for the existence of isotopes emerged from two independent lines of research, the first being the study of radioactivity. By 1910 it had become clear that certain processes associated with radioactivity, discovered some years before by Henri Becquerel, could transform one element into another. In particular, ores of the radioactive elements uranium and thorium had been found to contain small quantities of several radioactive substances never before observed. These substances were thought to be elements and accordingly received special names. Uranium ores, for example, yielded “ionium,” and thorium ores gave “mesothorium.” Painstaking work completed soon afterward revealed, however, that ionium, once mixed with ordinary thorium, could no longer be retrieved by chemical means alone. Similarly, mesothorium was shown to be chemically indistinguishable from radium. As chemists used the criterion of chemical indistinguishability as part of the definition of an element, they were forced to conclude that ionium and mesothorium were not new elements after all, but rather new forms of old ones. Generalizing from these and other data, Frederick Soddy in 1910 observed that “elements of different atomic weights may possess identical (chemical) properties” and so belong in the same place in the periodic table. With considerable prescience, he extended the scope of his conclusion to include not only radioactive species but stable elements as well. A few years later, Soddy published a comparison of the atomic weights of the stable element lead as measured in ores rich in uranium and thorium, respectively. He expected a difference because uranium and thorium decay into different isotopes of lead. The lead from the uranium-rich ore had an average atomic weight of 206.08 compared to 207.69 for the lead from the thorium-rich ore, thus verifying Soddy's conclusion. The unambiguous confirmation of isotopes in stable elements not associated directly with either uranium or thorium followed a few years later with the development of the mass spectrograph by Francis William Aston. His work grew out of the study of positive rays (sometimes called canal rays), first discovered in 1886 by Eugen Goldstein and soon thereafter recognized as beams of positive ions. As a student in the laboratory of J.J. Thomson, Aston had learned that the gaseous element neon produced two positive rays. The ions in the heavier ray had masses about two units, or 10 percent, greater than the ions in the lighter ray. To prove that the lighter neon had a mass very close to 20 and that the heavier ray was indeed neon and not a spurious signal of some kind, Aston had to construct an instrument that was considerably more precise than any other of the time. By 1919 he had done so and convincingly argued for the existence of neon-20 and neon-22. Information from his and other laboratories accumulated rapidly in the ensuing years and by 1935 the principal isotopes and their relative proportions were known for all but a handful of elements. LISTENING 1. You are going to listen to the staff report “IAEA Welcomes US Contribution of $50 million to Nuclear Fuel Bank”. Mind the following proper names: IAEA George Bush IAEA Director General Dr. Mohamed ElBaradei Nuclear Threat Initiative (NTI) NTI advisor Warren Buffett Former US Senator Sam Nunn, Co-Chairman of the NTI Angarsk
2. Listen to the staff report “IAEA Welcomes US Contribution of $50 million to Nuclear Fuel Bank”. Note only the essential details of what you hear: 1. The IAEA … 2. US President George Bush … 3. Dr. Mohamed ElBaradei said that … 4. The US contribution … 5. Former US Senator Sam Nunn, Co-Chairman of the NTI … 6. An IAEA-controlled fuel bank … 7. The law signed on 26 December … 8. The concept of a multilateral LEU supply bank … 9. A Russian proposal … 10. A German plan … 11. The establishment of a nuclear fuel supply system … 12. Enriched uranium provides …
3. Listen to the staff report again and complete the gaps in sentences below with the correct word or phrase you hear: 1. The IAEA has recognized a recent _______________ allocation by the US Congress for purposes of a nuclear fuel reserve under the auspices of the Agency. 2. "At the core of such mechanisms will be _______________, under IAEA auspices. 3. I also have welcomed _______________ for a fuel bank under IAEA control and a German initiative calling for the creation of an international enrichment centre, open to participation by all interested States. 4. This contribution was made by _______________ Warren Buffett in September 2006 with the stipulation that one or more IAEA Member States contribute an additional $100 million (or low-enriched uranium [LEU] equal in value) to the reserve. 5. An IAEA-_______________ is essential to reducing global nuclear dangers because the same nuclear enrichment technology that is used to make nuclear reactor fuel can also be used to make material for a nuclear weapon. 6. Assurances of supply of nuclear fuel, including _______________ (or banks), could provide States confidence in obtaining nuclear fuel for electricity generation and protect against disruption of supply for political reasons. 7. The risk of such _______________ could possibly dissuade countries from initiating or expanding nuclear power programmes or create vulnerabilities in the security of fuel supply that might in turn drive States to invest in national uranium enrichment capabilities with possible additional proliferation risks. 8. The plant would be financed by countries who would act _______________ of the plant´s nuclear fuel. 9. As an increasing number of nations plan for the development of _______________, concern has grown over the potential for diversion of nuclear material and technology from peaceful to military use. 10. Providing a _______________ to nations with a burgeoning nuclear power programme eases the economic cost and nuclear weapons-related risks intrinsic with building enrichment capabilities.
4. Listen to the staff report “IAEA Welcomes US Contribution of $50 million to Nuclear Fuel Bank” again and retell it.
PRESENTATION Make up a presentation “ATOMIC STRUCTURE OF MATTER” (See appendix 4)
SECTION 2 NUCLIDES LEAD-IN Define the following terms. a) Enriched uranium b) Depleted uranium
3. Give definition to the following terms:
a) nuclide b) atomic number c) mass number
4. The word ‘isotope’ comes from the Greek «isotopos» that means ‘the same place’. How can you explain this meaning? READING TEXT 1 Nuclides
Nuclides are characterized by the mass number (A) and the atomic number (Z). To be regarded as distinct a nuclide must have energy content sufficient for a measurable lifetime, usually more than 10−10 second. The term nuclide is not synonymous with isotope, which is any member of a set of nuclides having the same atomic number but differing mass number. Chlorine-37, the nucleus of which consists of 17 protons and 20 neutrons, is a different nuclide from sodium-23 (nucleus of 11 protons and 12 neutrons) or chlorine-35(nucleus of 17 protons and 18 neutrons). Nuclear isomers, which have the same number of protons and neutrons but differ in energy content and radioactivity, are also distinct nuclides.
Nuclides are associated with radioactive decay and may be stable or unstable species. About 1,700 nuclides are known, of which about 300 are stable and the rest radioactive. More than 200 of the stable nuclides were discovered by the British physicist Francis William Aston using his new invention of the mass spectrograph. The total number of protons in the nucleus of an atom is called the atomic numberof the atom and is given the symbol Z. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The mass numberof the nucleus is the total number of nucleons, that is, protons and neutrons in the nucleus. The mass number is given the symbol A and can be found by the equation Z + N = A. Each of the chemical elements has a unique atomic number because the atoms of different elements contain a different number of protons. The atomic number of an atom identifies the particular element. Each type of atom that contains a unique combination of protons and neutrons is called a nuclide.Not all combinations of numbers of protons and neutrons are possible, but about 2500 specific nuclides with unique combinations of neutrons and protons have been identified. Each nuclide is denoted by the chemical symbol of the element with the atomic number written as a subscript and the mass number written as a superscript, as shown in Figure 2. Because each element has a unique name, chemical symbol, and atomic number, only one of the three is necessary to identify the element. For this reason nuclides can also be identified by either the chemical name or the chemical symbol followed by the mass number (for example, U-235 or uranium-235). Another common format is to use abbreviation of the chemical element with the mass number superscripted (for example, 235U). In this handbook the format used in the text will usually be the element's name followed by the mass number. In equations and tables, the format in Figure 2 will usually be used. TEXT 2 Chart of the Nuclides The Chart of the Nuclides, like the Periodic Table, is a convenient format for presenting a large amount of scientific information in an organized manner.
A tabulated chart called the Chart of the Nuclides lists the stable and unstable nuclides in addition to pertinent information about each one. Figure 3 shows a small portion of a typical chart. This chart plots a box for each individual nuclide, with the number of protons (Z) on the vertical axis and the number of neutrons (N = A - Z) on the horizontal axis. The completely gray squares indicate stable isotopes. Those in white squares are artificially radioactive, meaning that they are produced by artificial techniques and do not occur naturally. By consulting a complete chart, other types of isotopes can be found, such as naturally occurring radioactive types (but none are found in the region of the chart that is illustrated in Figure 3). Located in the box on the far left of each horizontal row is general information about the element. The box contains the chemical symbol of the element in addition to the average atomic weight of the naturally occurring substance and the average thermal neutron absorption cross section, which will be discussed in a later module. The known isotopes (elements with the same atomic number Z but different mass number A) of each element are
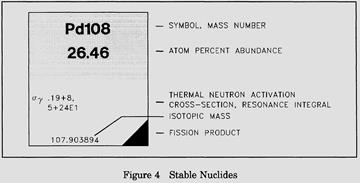
For the stable isotopes, in addition to the symbol and the atomic mass number, the number percentage of each isotope in the naturally occurring element is listed, as well as the thermal neutron activation cross section and the mass in atomic mass units (amu). A typical block for a stable nuclide from the Chart of the Nuclides is shown in Figure 4.
TEXT 3 Read the following definitions and decide what they mean. Share your ideas with other students in your group. Use a dictionary if necessary. Then enlarge the list of definitions and ask your groupmates to guess what they mean. Example Plumbing fixture consisting of a water basin fixed to a wall or floor and having a drainpipe is a sink.
1. A body that absorbs all radiation and radiates it all away is … 2. A quantum of electromagnetic radiation. An elementary particle that is its own antiparticle is … 3. A physical mechanism that transports heat and energy as electromagnetic waves is … 4. The force on a positive test charge per Coulomb due to other electrical charges is … 5. A property of a substance showing how well it radiates is … 6. The ability of a system to do work is … 7. The force on a moving positive test charge, per Coulomb, from magnets or moving charges is … 8. The law of physics that says the energy of a closed system doesn’t change unless external influences act on the system is … 9. ……………………………………………………………………………… 10. ………………………………………………………………………….. 11. ………………………………………………………………………….. 12. ………………………………………………………………………….. 13. …………………………………………………………………………..
LISTENING 1. You are going to listen to the staff report “Board Completes Discussions on Safety, Technology, and Verification“. Mind the proper names: Director General Dr. Mohamed ElBaradei the Board Integrated Regulatory Review Service (IRRS) Additional Protocol agreement with Swaziland Democratic People´s Republic of Korea (DPRK) Chairman Milenko E. Skoknic the Non-Proliferation Treaty (NPT) safeguards agreement 2. Listen to the staff report “Board Completes Discussions on Safety, Technology, and Verification“. Note only the essential details of what you hear: 1. The IAEA Board of Governors concluded … 2. Nuclear Safety Review and Nuclear Technology Review reports … 3. IRRS will assist Member States … 4. On the topic of nuclear science and technology … 5. Board members emphasized … 6. FAO/IAEA partnership … 7. Additional Protocol agreement with Swaziland … 8. Chairman Milenko E. Skoknic … 9. Non-Proliferation Treaty (NPT) … 10. Dr. ElBaradei … 3. Listen to the staff report again and complete the gaps in sentences below with the correct word or phrase you hear: 1. …the Agency and its Member States, focusing primarily on the IAEA´s activities related to nuclear science, __________. 2. Both documents, __________, comprise comprehensive reports that are assembled from comments provided by Member States along with input from the Agency. 3. Several conclusions were drawn regarding the __________ in nuclear safety and waste management. 4. Board members commended the Agency for its efforts __________ associated with nuclear power, noting the IAEA´s work in applying safety standards, promoting safety Conventions, and the recent establishment of the Integrated Regulatory Review Service (IRRS). 5. While members cited the importance of nuclear power as a possible method to meet growing __________, they also raised concerns over rising uranium prices and called on the IAEA to examine issues related to its mining. 6. Chairman Skoknic emphasized the "continued need for negotiation and dialogue among all parties involved covering all relevant issues" as necessary to achieve a long-term solution to the __________. 7. The three-day meeting was held in Vienna __________.
PRESENTATION Make up a presentation “NUCLIDES” (See appendix 4) SECTION 3 MASS DEFECT LEAD-IN Read the following text and do the tasks below (a, b). Careful measurements have shown that the mass of a particular atom is always slightly less than the sum of the masses of the individual neutrons, protons, and electrons of which the atom consists. The difference between the mass of the atom and the sum of the masses of its parts is called the mass defect (∆m). The mass defect can be calculated using Equation (1-1). In calculating the mass defect it is important to use the full accuracy of mass measurements because the difference in mass is small compared to the mass of the atom. Rounding off the masses of atoms and particles to three or four significant digits prior to the calculation will result in a calculated mass defect of zero.
a) Match the symbols with their meanings and explain the formula:
∆m mass of a proton (1.007277 amu) mp mass number (number of nucleons) mn mass of nuclide A/Z X (amu) me mass defect (amu) matom atomic number (number of protons) Z mass of an electron (0.000548597 amu) A mass of a neutron (1.008665 amu)
b) Calculate the mass defect for lithium-7. The mass of lithium-7 is 7.016003 amu.
READING TEXT 1 Example: A rusting nail Question: A 50-gram iron nail is left in a cup of water until it turns entirely to rust. The energy released is about 0.5 MJ (megajoules). In theory, would a sufficiently precise scale register a change in mass? If so, how much? Solution: The energy will appear as heat, which will be lost to the environment. So the total mass plus energy of the cup, water, and iron will indeed be lessened by 0.5 MJ. (If it had been perfectly insulated, there would have been no change, since the heat energy would have been trapped in the cup.) Converting to mass units, we have
so the change in mass is too small to measure with any practical technique. This is because the square of the speed of light is such a large number in metric units. In the example we tacitly assumed that the increase in mass would show up on a scale, i.e. that its gravitational attraction with the earth would increase. Strictly speaking, however, we have only proven that energy relates to inertial mass, i.e. to phenomena like momentum and the resistance of an object to a change in its state of motion. Even before Einstein, however, experiments had shown to a high degree of precision that any two objects with the same inertial mass will also exhibit the same gravitational attractions, i.e. have the same gravitational mass. For example, the only reason that all objects fall with the same acceleration is that a more massive object’s inertia is exactly in proportion to the greater gravitational forces in which it participates. We therefore conclude that energy participates in gravitational forces in the same way mass does. The total gravitational attraction between two objects is proportional not just to the product of their masses, m 1 m 2, as in Newton’s law of gravity, but to the quantity (m 1+ E 1)(m 2+ E 2). (Even this modification does not give a complete, self-consistent theory of gravity, which is only accomplished through the general theory of relativity.)
Adjectives/ adverbs at first transformed strange, unusual exact, correct common, usual Nouns surrounding conditions change of velocity change movement rule, regulation, principle 1.5. Bring your own examples to illustrate:
1. All energy is equivalent to mass 2. Energy participates in gravitational forces 3. Creation and destruction of particles TEXT 2 You are going to read about the examples of mass–energy equivalence. Six phrases have been removed from the text. Choose from the sentence A – G the one which fits each gap (1 – 6). There is one extra phrase which you don’t need to use. A after cooling (the heat, light and radiation in this case carried the missing gram of mass) B However, Einstein’s equations show that all energy has mass. C Whenever energy is added to a system. D when testing Einstein’s theory of general relativity. E and therefore the potential energy, in principle. F which is sometimes called the "active energy". G when energy of any kind is added to a resting body.
TEXT 3 Electromagnetic Rest Mass
For questions 1-7, read the text below and decide which answer (A, B, C or D) best fits each gap.
There were many (1)__________ in the 19th and the beginning of the 20th century - like those of J. J. Thomson (1881), Oliver Heaviside (1888), George Frederick Charles Searle (1896), - to understand how the mass of a(n) (2)__________ object varied with the velocity. Because the electromagnetic field carries part of the momentum of a moving charge, it was suspected that the mass of an electron would vary with velocity near the speed of light. Following Searle (1896), Wilhelm Wien (1900), Max Abraham (1902), and Hendrik Lorentz (1904) concluded that the velocity dependant electromagnetic mass of a body (3)_______ is m = (4 / 3)E / c2. According to them, this relation applies to the complete mass of bodies, because any form of inertial mass was considered to be of electromagnetic (4)________. Wien went on by stating, that if it is assumed that gravitation is an electromagnetic effect too, than there has to be a(n) (6)________ proportionality between (electromagnetic) inertial mass and (electromagnetic) gravitational mass. To explain the stability of the matter-electron configuration, Poincarй in 1906 introduced some sort of pressure of non-electrical nature, which contributes the amount − (1 / 3)E / c2 to the mass of the bodies, and (7)_________ the 4/3-factor vanishes.
1 A attempts B thoughts C ideas D works 2 A charged B loaded C inspired D forced 3 A at rest B in peace C in calmness D at repose 4 A origin B spring C root D basis 5 A origin B spring C root D basis 6 A strict B severe C austere D stringent 7 A therefore B naturally C moreover D even Albert Einstein
LISTENING 1. You are going to listen to the staff report “Joint Actions Helping to Bolster Nuclear Security China Links Up With IAEA on Nuclear Security for Summer Games “. Mind the proper names: Anita Nilsson, Director of the IAEA´s Office of Nuclear Security China Atomic Energy Authority (CAEA) the 2006 FIFA World Cup the 2007 Pan American Games Beijing 2. Speed listening. Note only the essential details of what you hear: 1. The Olympics………………………. 2. China and the IAEA…………………….. 3. A training workshop……………………….. 4. Anita Nilsson said……………………………… 5. IAEA´s work in Beijing……………………… 6. Chinese authorities………………………… 7. Nuclear security measures………………………………… 8. Help to Member states…………………………………………
3. General information: Complete the chart with the basic ideas:
4. Gap filling: Listen once again and complete the gaps in the summary of the passage below with the correct word or phrase you hear:
On the threshold of the Summer Olympic Games Chine and the IAEA are working together at strengthening _________________ and minimizing threats. For that purpose _______________ was held in Beijing. The IAEA’s role was to assist in integrating __________________into the existing Chinese security system. The spheres which are covered by the conducted advisory missions and training exercises are __________________. The IAEA has already rendered technical assistance to different international public events among which are ____________________. Member States of the IAEA get comprehensive help from the Office of Nuclear Security in form of __________________. This is vitally important for protecting ______________________ and fighting against___________________________.
5. Decide whether these statements are true, false or the information is not given:
1. China and the IAEA are creating country’s new security plan. 2. Work of Chine with the IAEA’s specialists has been lasting for one and a half year. 3. The IAEA’s work in Beijing is provided only for intelligence community. 4. A training workshop was widely covered in the press. 5. The China Atomic Energy Authority is the main coordinator of activities. 6. The IAEA is now actively working with Peru on arranging another training seminar for security officers. 7. Several Member States that need help in the formation of security planning have recently signed agreements with the IAEA. PRESENTATION Make up a presentation “MASS DEFECT” (See appendix 4) SECTION 4 BINDING ENERGY LEAD-IN READING TEXT 1 Before reading the text below, work in small groups (2-3 students) and decide whether the statements 1-5 are true or false using your knowledge of the subject. Then read the text and check your guesses. a. Binding energy can not be considered equivalent to the mass defect. b. All the electrons are equally bound in the atom. c. Ionization is the process of removing an electron from an atom. d. Atom can stay in an excited state for a long time without changing its orbit. e. The only difference between x-rays and γ-rays is intensity of electromagnetic radiation. Overview of Binding Energy The loss in mass, or mass defect, is due to the conversion of mass lo binding energy when the nucleus is formed. Binding energy is defined as the amount of energy that must be supplied to a nucleus to completely separate its nuclear particles (nucleons). It can also be understood as the amount of energy that would be released if the nucleus was formed from the separate particles. Binding energy is the energy equivalent of the mass defect. Since the mass defect was converted lo binding energy (BE) when the nucleus was formed, it is possible to calculate the binding energy using a conversion factor derived by the mass-energy relationship from Einstein's Theory of Relativity. Einstein's famous equation relating mass and energy is E = mc² where с is the velocity of light (C = 2.998 x 10*8 m/sec). The energy equivalent of 1 amu can be determined by inserting this quantity of mass into Einstein's equation and applying conversion factors.
Conversion Factors:
Since 1 amu is equivalent to 931.5 MeV of energy, the binding energy can be calculated using Equation (1-2).
Example: Calculate the mass defect and binding energy for uranium-235. One uranium-235 atom has a mass of 235.043924 amu.
Solution: Step 1: Calculate the mass defect using Equation (1-1).
Step 2: Use the mass defect and Equation (1-2) to calculate the binding energy.
Energy Levels of Atoms: The electrons that circle the nucleus move in fairly well-defined orbits. Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88.000 eV is required to remove the innermost electron. The process of removing an electron from an atom is called ionization, and the energy required to remove the electron is called the ionization energy. In a neutral atom (number of electrons = Z) it is possible for the electrons to be in a variety of different orbits, each with a different energy level. The state of lowest energy is the one in which the atom is normally found and is called the ground state. When the atom possesses more energy than its ground state energy, it is said to be in an excited state.
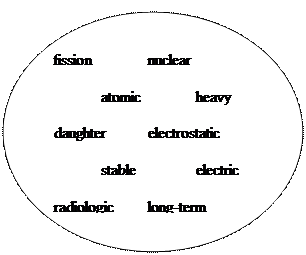
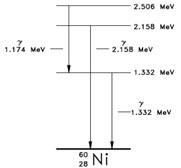
Energy Levels of the Nucleus: The nucleons in the nucleus of an atom, like the electrons that circle the nucleus, exist in shells that correspond to energy states. The energy shells of the nucleus are less defined and less understood than those of the electrons. There is a state of lowest energy (the ground state) and discrete possible excited states for a nucleus. Where the discrete energy states for the electrons of an atom are measured in eV or keV, the energy levels of the nucleus are considerably greater and typically measured in MeV. A nucleus that is in the excited state will not remain at that energy level for an indefinite period. Like the electrons in an excited atom, the nucleons in an excited nucleus will transition towards their lowest energy configuration and in doing so emit a discrete bundle of electromagnetic radiation called a gamma ray (γ-ray). The only differences between x-rays and γ-rays are their energy levels and whether they are emitted from the electron shell or from the nucleus. The ground slate and the excited slates of a nucleus can be depicted in a nuclear energy-level diagram. The nuclear energy-level diagram consists of a slack of horizontal bars, one bar for each of the excited states of the nucleus. The vertical distance between the bar representing an excited state and the bar representing the ground state is proportional to the energy level of the excited state with respect to the ground slate. This difference in energy between the ground state and the excited state is called the excitation energy of the excited state. The ground state of a nuclide has zero excitation energy. The bars for the excited states are labeled with their respective energy levels. Figure 7 is the energy level diagram for nickel-60.
1.2. Find antonyms to the following words in the text above:
TEXT 2 Nuclear Processes Work in two groups. Group I 1. You are going to read the text about Nuclear fission. Seven phrases have been removed from the text. Choose from the sentence a-h the one which fits each gap (1 – 7). There is one extra phrase which you don’t need to use.
a. and therefore are the most stable b. because this allows one product to be closer to the energetic minimum near mass 60 u (only a quarter of the average fissionable mass) c. once a fuel element has been used -
2. Match the notions and their meanings: 1. chain reaction a) the compound D2O composed of deuterium and oxygen —called also deuterium oxide 2. isotope b) something that is built, installed, or established to serve a particular purpose 3. gamma ray c) a source of danger 4. heavy water d) a series of events so related to each other that each one initiates the next 5. hazard e) a photon emitted spontaneously by a radioactive substance 6. half-life f) the time required for half of the atoms of a radioactive substance to become disintegrated 7. facilities g) any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties
3. Find words in the text above which mean the following:
Verbs
1. to clash 2. to initiate 3. to generate/ cause/ prompt 4. to control/ command/ restrict 5. to change/ alter/ modify 6. to compose/ compile 7. to release/expel/ eject 8. to take in/ consume/ eat or drink
4. Make a list of collocations with the words below and use them in sentences of your own:
Group II 1. You are going to read the text about nuclear fusion. Six phrases have been removed from the text. Choose from the sentence a-g the one which fits each gap (1 – 6). There is one extra phrase which you don’t need to use. 1. accelerated to high speeds (that is, heated to thermonuclear temperatures) 2. production of the heaviest elements absorbs energy 3. research into fusion for military purposes began in the early 1940s 4. where the extreme power of a fission bomb is necessary to begin the process 5. therefore are the most stable 6. in which one daughter nucleus has a mass of about 90 to 100 u and the other the remaining 130 to 140 u 7. which are themselves millions of times more energetic than chemical reactions Nuclear Fusion In physics and nuclear chemistry, nuclear fusion is the process by which multiple atomic particles join together to form a heavier nucleus. It is accompanied by the release or absorption of energy. Iron and nickel nuclei have the largest binding energies per nucleon of all nuclei and 1)________. The fusion of two nuclei lighter than iron or nickel generally releases energy while the fusion of nuclei heavier than iron or nickel absorbs energy; vice-versa for the reverse process, nuclear fission. Nuclear fusion occurs naturally in stars. Artificial fusion in human enterprises has also been achieved, although not yet completely controlled. Building upon the nuclear transmutation experiments of Ernest Rutherford done a few years earlier, fusion of light nuclei (hydrogen isotopes) was first observed by Mark Oliphant in 1932, and the steps of the main cycle of nuclear fusion in stars were subsequently worked out by Hans Bethe throughout the remainder of that decade. 2)__________, as part of the Manhattan Project, but was not successful until 1952. Research into controlled fusion for civilian purposes began in the 1950s, and continues to this day. Fusion reactions power the stars and produce all but the lightest elements in a process called nucleosynthesis. Whereas the fusion of light elements in the stars releases energy, 3)_________. When the fusion reaction is a sustained uncontrolled chain, it can result in a thermonuclear explosion, such as that generated by a hydrogen bomb. Reactions which are not self-sustaining can still release considerable energy, as well as large numbers of neutrons. Research into controlled fusion, with the aim of producing fusion power for the production of electricity, has been conducted for over 50 years. It has been accompanied by extreme scientific and technological difficulties, and as of yet has not been successful in producing workable designs. As of the present, the only self-sustaining fusion reactions produced by humans have been produced in hydrogen bombs, 4)_________. While some plans have been put forth to attempt to use the explosions of hydrogen bombs to generate electricity (e.g. PACER - programmed analysis and calculation of equipment reliability), none of these have ever moved far past the design stage. It takes considerable energy to force nuclei to fuse, even those of the lightest element, hydrogen. This is because all nuclei have a positive charge (due to their protons), and as like charges repel, nuclei strongly resist being put too close together. 5)___________, however, they can overcome this electromagnetic repulsion and get close enough for the strong nuclear force to be active, achieving fusion. The fusion of lighter nuclei, creating a heavier nucleus and a free neutron, will generally release more energy than it took to force them together-an exothermic process that can produce self-sustaining reactions. The energy released in most nuclear reactions is much larger than that in chemical reactions, because the binding energy that holds a nucleus together is far greater than the energy that holds electrons to a nucleus. For example, the ionization energy gained by adding an electron to a hydrogen nucleus is 13.6 electron volts - less than one-millionth of the 17 MeV released in the D-T (deuterium-tritium) reaction shown to the top right. Fusion reactions have an energy density many times greater than nuclear fission-that is, per unit of mass the reactions produce far greater energies, even though individual fission reactions are generally much more energetic than individual fusion reactions – 6)________. Only the direct conversion of mass into energy, such as with collision of matter and antimatter, is more energetic per unit of mass than nuclear fusion. 2. Match the notions and their meanings: 1. nucleona) a fundamental form of energy observable in positive and negative forms that occurs naturally (as in lightning) or is produced (as in a generator) and that is expressed in terms of the movement and interaction of electrons 2. nucleosynthesis b) a proton or neutron especially in the atomic nucleus 3. self-sustaining c) an uncharged elementary particle that has a mass nearly equal to that of the proton and is present in all known atomic nuclei except the hydrogen nucleus 4. electricity d) th
|
|||||||||||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2016-08-01; просмотров: 530; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.33 (0.014 с.) |

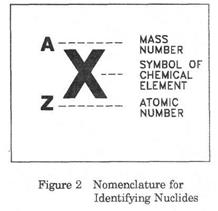 Nuclides are commonly expressed in the form A/ZX, where A denotes the total number of protons and neutrons, Z represents the number of protons, and the difference between A and Z is the number of neutrons. Thus 37/17Cl signifies chlorine-37.
Nuclides are commonly expressed in the form A/ZX, where A denotes the total number of protons and neutrons, Z represents the number of protons, and the difference between A and Z is the number of neutrons. Thus 37/17Cl signifies chlorine-37.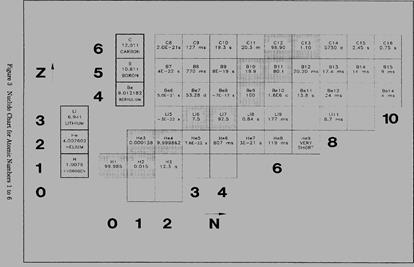 listed to the right.
listed to the right.
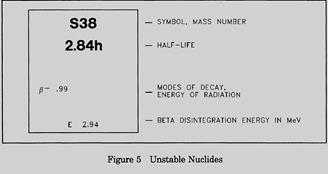 For unstable isotopes the additional information includes the half life, the mode of decay (for example, β, α), the total disintegration energy in MeV (million electron volts), and the mass in amu when available. A typical block for an unstable nuclide from the Chart of the Nuclides is shown in Figure 5.
For unstable isotopes the additional information includes the half life, the mode of decay (for example, β, α), the total disintegration energy in MeV (million electron volts), and the mass in amu when available. A typical block for an unstable nuclide from the Chart of the Nuclides is shown in Figure 5.




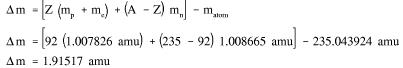
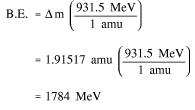
 An atom cannot stay in the excited state for an indefinite period of time. An excited atom will eventually transition to either a lower-energy excited state, or directly to its ground state, by emitting a discrete bundle of electromagnetic energy called an x-ray. The energy of the x-ray will be equal to the difference between the energy levels of the atom and will typically range from several eV to 100.000 eV in magnitude.
An atom cannot stay in the excited state for an indefinite period of time. An excited atom will eventually transition to either a lower-energy excited state, or directly to its ground state, by emitting a discrete bundle of electromagnetic energy called an x-ray. The energy of the x-ray will be equal to the difference between the energy levels of the atom and will typically range from several eV to 100.000 eV in magnitude.