Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
The Russian metallurgist D. K. ChernovСодержание книги Поиск на нашем сайте
The father of the branch of science concerned with the changes in structure of steels was the Russian metallurgist Dmitry Konstantinovich Chernov (1839-1921). Investigating the properties of steel after heating to various temperatures, Chernov first established that at definite temperatures steel undergoes certain changes altering its structure and properties. These '"critical temperatures" characterized by internal changes in the steel are now known all over the world as the "Chernov points". One of these points called by Chernov point a, is notable for the fact that steel heated below this point (about 700°C) cannot be hardened no matter how rapidly it is cooled. Another point, b, is characterized by the fact that as soon as the temperature of the steel reaches it (800 to 850°C), the steel rapidly passes from the coarse crystalline into the fine crystalline state, in which it possesses the best mechanical properties. If the temperature is raised still further, the metal crystals begin to increase again in size, and the higher the temperature, the more rapidly they grow. The discovery of the critical points of steel was of very great importance for metallurgical theory and practice. Explaining the phenomena of tempering and hardening of steel and the structural changes taking place in steel when heated, it enabled accurate determination of the hardening temperatures and selection of favourable conditions of forging and other types of steel treatment, promoting improvement of its mechanical properties.
OXYGEN IN THE BESSEMER CONVERTER
Fundamentally, the addition of oxygen to the Bessemer air blast should have two major effects: (1) to increase the rate of oxidation because of the higher partial pressure of oxygen in the system; (2) to increase the amount of heat available to melt cold materials. The use of oxygen for enriching the blast of the basic Bessemer converter or for changing the nature of the converter blast altogether, became a very important development in steel works. Reduction of nitrogen content in Bessemer steel by oxygen enrichment alone reaches a limit at about.006 per cent nitrogen. Further it is necessary to remove all nitrogen from the blast but, at the same time, maintain a blast composition which will oxidize metalloids in the steel without generating so much heat that the converter tuyeres are burned out. This can be done by a mixed blast of oxygen and superheated steam. By this method steel containing less than.003 per cent nitrogen is produced. One potential source of difficulty in the oxygen-steam mixed blast process is the danger that condensate from the steam may attack the dolomite bottom of the converter. To avoid this, some designers recommend the use of thin copper tubes embedded in the bottom plate to act as protective liners for the tuyeres. Quite recently some experiments were made with the so-called "dual-blast" converter in which air is blown upward through ordinary tuyeres in the bottom while high-purity oxygen from the top is directed downward at low impact pressure onto the surface of the bath. This oxygen is intended not so much to penetrate the metal as to flood the whole atmosphere above the converter bath With oxygen, thus promoting high concentration and reactivity of iron oxide in the basic slag. By changing the number of open tuyeres in the bottom of the converter the ratio of oxygen going down to air going up can be varied within wide limits. The object of this departure is to develop a conversion process that will remove phosphorus from Thomas pig-iron before decarburization is complete. By passing oxygen downward onto the surface of the metal and the lime which are being agitated by the rising air blast, the necessary early liquefying of the lime is achieved and early dephosphorization takes place. OXYGEN ENRICHMENT IN THE BLAST FURNACE
Oxygen enrichment by itself has been considered of marginal advantage in the blast furnace except in the production of ferro-alloys. This is because a greatly increased tuyere zone temperature upsets the thermal balance and leads to some difficulty in avoiding the production of high silicon iron. It has been found, however, that oxygen-enriched blast combined with steam injection serves to drive the furnace faster and generate a gas of greater reducing power with no significant change in hearth zone temperature. Some plants currently practice routine oxygen enrichment of blast furnace air for the production of steelmaking iron. Approximately 65 tons of oxygen per day per furnace are required for each 1 per cent of enrichment. With an available oxygen supply of about 400 tons per day, it has been found advantageous to spread this amount over four operating furnaces rather than, to concentrate it on one furnace. The economic attractiveness of oxygen enrichment in the blast furnace is greatest under conditions of capacity operation when there is a strong demand for the additional iron which can be produced. The most promising use of blast furnace oxygen enrichment lies in ferro-alloy smelting. The high hearth temperature produced with oxygen favours the reduction of non-ferrous metal oxides and formation of their high melting point slag. OXYGEN FOR DIRECT REDUCTION OF IRON ORE
Basically the industry is still dependent on the units of production that characterized it in the early years of this century by-product coke ovens, blast furnaces, open hearths, blooming and secondary mills. Direct ore reduction processes may change this since they provide a potential replacement for the coke oven-blast furnace complex. The H -iron process is a typical example. This process consists in bringing into intimate contact, at about 900°F and 400 pounds per square inch, finely divided iron ore and preheated hydrogen gas. Pressurized hydrogen enters a large cylindrical reducing vessel at the bottom and in passing upward keeps the fine ore particles in a state of turbulent motion. In the reducer, hydrogen reacts with the preheated iron oxide to form reduced iron and water:
Fe2O3+3H2=2Fe+3H2O.
The gas leaving the top of the reactor consists of unreacted hydrogen and water vapour. Regenerated hydrogen, as well as fresh hydrogen to take the place of the hydrogen converted to water, is recycled to the reducer. The reduced iron product is briquetted, thermally passivated, and can then be charged directly to open hearth or electric furnaces to replace the scrap. The production of hydrogen for such a process may be accomplished by exothermic partial oxidation of natural gas of fuel oil. CRUCIBLE FURNACE
The crucible furnace is one of the oldest furnaces for melting non-ferrous metals, and it is being used a great deal at the present time. High carbon steel is made by melting scrap steel in a crucible too. The construction of a crucible furnace consists of a shell which is made of a steel plate about 3/16 in. thick. It rests on a plate made of cast iron. The legs support the furnace high enough from the floor to allow for draft and ashes. The grate works on hinges so that it can be raised and dropped. The grate is turned by the handle. The cover holds down the flame. The furnace should be connected by the flue with a chimney. The melting capacities of the crucible in one heat are from 20 to 300 lbs of brass or from 10 to 100 lbs of aluminium. The linings are made of brick and are applied about the same as in the cupola furnace. Sometimes the furnaces are set on the floor, but, in most cases, they are set in a pit because they are more easily operated in such a setting. Shavings and kindling are placed on the grate with about 50 lbs of coke on the kindling. After the coke bed is well lighted, one should build up the coke bed so that the top of the crucible, when put in, will be even with the top of the flue. The bottom of the furnace and grates should be kept free from ashes and cinders, or the draft will not get through, causing much trouble in getting the metal enough to run the castings. The crucible way is used for making high carbon steel and special steels. The percentage of carbon the scrap steel contains must be known. PORTABLE HARDNESS TESTER
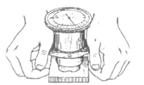
Fig.56. Portable Tester
In use, the portable tester is applied to the work, as shown in Fig. 56, and a handpiece is depressed, with the result that a diamond indentor is brought into contact with the surface. Continued movement of the handpiece causes the indentor to be pressed into the work under a pre-determined load for a maximum depth of 0.005 in. A reading which corresponds to the hardness value is then obtained on a large-diameter dial-type indicator. Scales of different colours are provided which give direct readings in Vickers pyramid, Brinell, and Rockwell values. The instrument is available in different types, one of which gives full scale readings for Vickers pyramid numbers from 80 to 1200, Brinell from 80 to 630, and Rockwell from 30 to 70. A second instrument gives full scale readings in Vickers pyramid numbers from 35 to 300. Another type provides readings of Rockwell A, B, and С values from 30 to 90, 40 to 100, and to 70. On the fourth hardness tester the full scale readings obtainable range from 80 to 1200 Vickers pyramid, 80 to 420 Brinell, 35 to 99 Rockwell B, and 20 to 70 Rockwell C.
|
||||
|
Последнее изменение этой страницы: 2016-08-12; просмотров: 444; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.227.105.110 (0.007 с.) |