Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
I. Answer the questions in written form and make up a dialogue using some of them.Содержание книги
Поиск на нашем сайте
1. What does aluminium look like?
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 2. Where does aluminium occure? ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 3. What makes aluminium very suitable for the bodies of vehicles?
-------------------------------------------------------------------------------------------------------------------------------------------------------------------=-------------------------------------------------------------------- 4. Why is aluminium used for the heat insulation of houses?
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 5. What important aluminium alloys do you know?
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 6. Where is aluminium used in every-day life?
-------------------------------------------------------------------------------------------------------------------- -------------------------------------------------------------------------------------------------------------------- 7. What is the meaning of the word ‘alloy’?
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 8. What are the properties of aluminium alloys? ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- II. Translate into Ukrainian in written form. Consult various kinds of dictionaries. Metal Fatigue Fatigue in metals occurs when the cohesion in metal (or other material structure) is gradually broken down by repeated stresses. Finally the cohesion gives way and a fracture occurs. Cohesion is the force which tends to hold a body together and is greatly reduced by repeated stresses and then a break takes place. Most fractures in machine parts and structures are the result of fatigue, if they were subjected to repeated loading. The trouble is, of course, that it is not possible to know ahead when a part will fail because of fatigue, there are no warnings. Nothing can be seen with the naked eye. Fatigue can be predicted only from experimental data. For this reason and also because both tensile and bending stresses involve complex problems, new laboratory techniques have been developed and fatigue testing has become almost a separate and a most important field.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ Text 6 NON-FERROUS METALS AND THEIR ALLOYS (Part I) COPPER, TIN, BRONZE Copper (Cu) is found in nature in the form of ores but it is sometimes found in pure state. Pure copper is of reddish colour and it has corrosion resistant qualities. Copper is the best conductor of electricity. It is used a great deal for electrical wiring and cables. It is a good conductor and it is surpassed only by silver for conductivity of electricity and the making of electrical apparatus is the chief use for copper, e.g. telephone and telegraph cables; electric wiring; parts of. dynamos and electric motors. Three important copper alloys are brass, bronze and cupro-niekel (75% copper + 25% nickel), which is used for the present “silver” coins. Tin (Sn) is a silvery metal which is not corroded by air. The chief use is for making tin plate, i.e. sheetiron coated with tin. Tin plate is suitable for cans in which acid fruit and other food-stuffs are packed because tin is not attacked by weak acids. For good containers, iron is coated with tin instead of zinc because tin is not subject to attack by acids in food. An alloy of copper and tin (80% + 20% respectively) is called bronze. Bronze is very tough; it withstands shock; it wears well. Bronze is used for making ship propellers, parts of machinery, subject to hard wear, and for doors and windows. Words and word combinations to surpass – перевищувати brass – латунь (жовта мідь) cupro-niekel – мельхіор, міднонікелевий сплав tin (Sn) = stannum – олово sheetiron – листове залізо tin is not subject to attack – на олово не впливають respectively – відповідно subject to hard wear – які зазнають серйозного зношування (Part II) LEAD, SOLDER, ZINK Lead (Pb) is now a very expensive metal. At one time it was widely used for roofing and for water piping because of its softness and resistance to corrosion, but copper and iron have taken its place. Lead is a gray malleable metal which melts at 327°C, which is low for a metal. Lead is still used for roofing and for making waste pipes and sink traps because it is easily bent into shape; storage battery (accumulator) plates; cable sheaths; storage tanks for sulphuric acid; lead shot; solder; screens to stop harmful radiation from radioactive substances. Other lead alloy is type metal (lead + tin + bismuth (Bi) + cadmium (Сd)). Lead monoxideis used for making glass that is brilliant and sparking, i.e. decorative tableware. Solder (33% tin + 67% lead) has lower melting point than either tin or lead and therefore is used for making repairs. Zink is a moderately hard grey metal which acquires a protective coating of zink oxide on its surface. Its colour is yellow when hot and white when cold. It can be turned on a lathe and pressed into shape. Zink oxide is used in paints because it is non-poisonous and is not discoloured by hydrogen sulphide. It has a soothing effect upon the skin and is used in ointments and lotions. It is added to rubber for making racing motor tyres. Zink is used in the making of dry batteries and in the process of galvanizing. In this, iron is dipped into molten zink, which forms a protective layer on its surface. Galvanized iron is used in sheets for roofing and also for buckets and dustbins. Words and word combinations solder – припой piping – трубопровід malleable – ковкий sink traps – тут: дренажні труби cable sheaths – тут: захисний кожух sulphuric acid – сірчана кислота shot – тут: дріб type metal – гарт (типографський сплав) tableware – столовеприладдя (ложки, виделки і т. п.) for making repairs – для ремонту hydrogen sulphide – сульфід водню an ointment – мазь to turn on – тут: обточувати to press into shape – пресувати по формі
EXERCISES I. Give the Ukrainian equivalents 1- 5 (Part I), 6 – 10 (Part II).
II. State the part of speech of the words in the brackets. Fill the blanks with proper words. 1. His................................ as head of the department was not unexpected. The next thing to do was......................................... a time for the meeting. (to appoint, appointment) 2. Peace....................................... is supported by all the progressive people in the world. Don't try........................... that table, it is not..................................., it is fixed to the floor. (to move, movement, movable) 3. Your................................. at the meeting is absolutely necessary. Unfortunately we were not................................. at the lecture yesterday. The solution of the problem.................................. great difficulties. (presents, presence, present) 4. Vernadsky's theory of the biosphere.................................. that the problem of environmental protection have gone beyond national boundaries. The proportion of living matter is......................................... compared to the mass of the Earth. This discovery is very........................................ in its consequences. (significant, insignificant, signifies) III. Interpret the meaning of the following correlated terms and use 5 of them in the sentences of your own: 1) technique, technology, engineering ---------------------------------------------------------------------------------------------------------------------- 2) science, knowledge, learning ---------------------------------------------------------------------------------------------------------------------- 3) advance, progress ----------------------------------------------------------------------------------------------------------------------
1) ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 2) ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 3) ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 4) ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 5) ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- IV. Translate the sentences paying attention to the polysemy of the words in bold type.
1. We don't know the number (……………………..) of computer users in this country. 2. What is your telephone number (……………………..)? 3. As a rule programmers know a number of (……………………..)languages of programming. 4. A new processor was the “ Number (……………………..)One” of presentation. 5. The latest achievements in cybernetics were published in October number (……………………..)of a scientific journal. 6. To satisfy the increasing demands of users a new high-speed computer was designed (…………………………………..……..) not long ago. 7. A new school was designed (…………………………………..……..)by a famous architect. 8. The icebreaker was designed (……………………….……………..……..)for operation in Arctic waters. 9. It was designed (……………………..…….……..……..)to fulfill the task till the end of month. 10. This result was not designed (……………………………………………..…..……..).
Text 7 METAL JOINTS These joints are permanent. In soldering and welding heat used to achieve the joints. Rivets can also be used with sheet metal to effect a permanent joint. The decision on which method to use for metal jointing depends on the metal, the stress to which the joint will be subjected and the structure of work.
There are several ways to solder. For example, for difficult joints 'sweat' soldering is the best method. You need to coat both sides of the joint with a layer of solder then bring them together, heating carefully with a low flame whilst the joint is held firmly in place. Hard solder. This is similar to soft solder but involves much higher temperatures, typically +650°C. The result is a stronger joint. There are two types of hard solder: • Brazing - hardest solder with very high melting point (about 815°C), using alloy of copper and zinc. • Silver - hard solder with high temperatures (650-800°C), using an alloy of s copper and zinc. In both cases joints need to be held securely, often with wire. An oxy-acetylene welding torch will be used to provide the heat. The flame needs to be bushy rather than narrow so that the flux is not blown away from the joint.
When welding, a pool of molten metal is created by the flame and a filler rod is continually dipped into it. The filler rod is the same metal as those being joined and it melts into the joint, thereby filling it and fusing the metal together. In electric arc welding the filler rod is flux coated. EXERCISES
|
||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2016-08-12; просмотров: 74; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.191.205.110 (0.009 с.) |

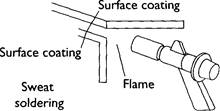 Soft solder. This is an alloy of tin and lead. It melts at +I85°C. As a jointing method it is easy and quick, particularly for brass, copper and metal sheet. The proportions of tin and lead in the solder should be varied with the task and metals involved. For example, if jointing sheet metal more lead needs to be used. This is because it melts at a higher temperature and so sets much harder. Conversely, if using solder for electronics more tin is required in the mix.
Soft solder. This is an alloy of tin and lead. It melts at +I85°C. As a jointing method it is easy and quick, particularly for brass, copper and metal sheet. The proportions of tin and lead in the solder should be varied with the task and metals involved. For example, if jointing sheet metal more lead needs to be used. This is because it melts at a higher temperature and so sets much harder. Conversely, if using solder for electronics more tin is required in the mix.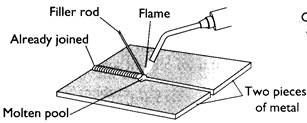 Welding. Welding is the act of fusing together two pieces of into one piece. It is done under very high temperatures using oxy-acetylene gas equipment or electric arc welding one.
Welding. Welding is the act of fusing together two pieces of into one piece. It is done under very high temperatures using oxy-acetylene gas equipment or electric arc welding one.