Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Indoor air pollution. Public health problemСодержание книги
Поиск на нашем сайте
Indoor air pollution consists of toxic gases or particles that can harm human health. We spend most of our time indoors surrounded by sources of air pollution: consumer products, gas appliances, building materials, cigarettes, and furniture can all contribute to the problem. U.S. EPA, 1987 ranked indoor air pollution 4th in cancer risk among the 13 top environmental problems analyzed. Indoor radon ranked first. Factors contributing to the high risk: 1) People are spending most of their time indoors (an average of 87% of their 24-hour day indoors). 2) Indoor air pollutant levels are often higher than those outdoors (formaldehyde, chloroform, and styrene, range from 2 to 50 times higher). 3) Exposure to pollutants such as environmental tobacco smoke and radon occurs almost entirely indoors. The amount of air pollution that we breathe is primarily determined by the indoor air! According to The world health report 2002 indoor air pollution is responsible for 2.7% of the global burden of disease. More than three billion people worldwide continue to depend on solid fuels, including biomass fuels (wood, dung, agricultural residues) and coal, for their energy needs. Cooking and heating with solid fuels on open fires or traditional stoves results in high levels of indoor air pollution. Indoor smoke contains a range of health-damaging pollutants, such as small particles and carbon monoxide, and particulate pollution levels may be 20 times higher than accepted guideline values. Yet, the toxic emissions from many of these sources are not controlled or are only partially controlled by state or local laws. Primary air pollutants: 5 types of materials released directly into the atmosphere in a harmful form: Carbon monoxide (CO), Particulate matter (PM), Sulfur dioxide (SO2), Nitrogen oxides (NOx) and Volatile organic compounds (hydrocarbons). Concentration of air pollutants may be expressed in: mg/m3, ppm = parts per million, or 10-6 ppb = parts per billion, or 10-9
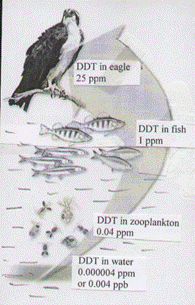
Biomagnification – the process whereby the concentration of a fat-soluble contaminant increases as it passes up the food chain. With which consumption step, the concentration of the contaminant in the consumer biomass is magnified (from 10 ppm to 106 ppm). Magnification ratio (MR) – ratio of a real air pollutant concentration to maximum admissible concentration (MAC). It shows how many times real concentration of pollutant in the air exceeds standard level. Example: Magnification ratio of CO equals 2: MR(CO) = Toxic substances (pesticides, herbicides, mercury, lead etc) are able to accumulate in the cells and tissues of any organism. In marine systems concentration of toxins in organisms is increasing as we move up the food chain from producers to consumers: water (sea, lake) à phytoplankton à zooplankton à fish à fish-eating birds. Bioaccumulation Factor (BAF) is the ratio of a toxic substance’s concentration in a given organism to that in the surrounding medium (water). Biomagnification Factor (BMF) is the ratio of concentration of toxic substance in a higher level of the food chain to that in the lower level of the same food chain.
4. The nature, source & the effects of acid rain. Acid rain is rain or any other form of precipitation that is unusually acidic. “Acid rain” is a by-product of modern atmospheric pollution – secondary pollutant. The pH of acid rain is bellow 5.6. Acid rain does not occur as rain; it also occurs as mist, fog, sleet, snow, gas and dry dust particles. It caused by pollution in the air, which comes from burning fossil fuels such as oil and coal. Sulfur dioxide (SO2), Nitrogen oxide (NOx) and polycyclic aromatic hydrocarbons (PAH) are some of the main compounds that result from burning fossil fuels. They combine in the air to form a chemical mixture, which eventually returns to earth as acid rain. Chemical reactions: SO3 + H2O → H2SO4 NOx + H2O → HNOx Some of this pollution falls close to where it came from; however, weather systems can also carry some of this pollution more than 2.000 km from its source before it falls back down to earth. Acid rain is an international problem. For ex, Sweden has suffered badly from acid rain damage: 90% of SO2 that falls on the country every year comes from other countries. The acidity of rain can be between 5 and 2, and 1000 times more acidic than is natural. In some of the worst cases, the rain is as acidic as vinegar (pH 2,7), lemon juice (pH 2) and battery acid (pH 1). Rainfall in unpolluted areas has pH ~ 5.6. Sources of acid rain. Industries can contribute to acid rain if: - They burn fossil fuels: for ex, the oil and coal that industries use for energy and heating contributes about 17% of the UK’s sulfur dioxide air emissions. - They use motor vehicles for goods transportation or other business: the exhaust fumes from motor vehicles produce 45% of the UK’s NOx air emissions and 30% of its are hydrocarbons. - They use electricity from power stations, which use fossil fuels; 65% of SO2 emissions come from power stations. - They have older style technology: much of this uses fuels with high sulfur and nitrogen contents and does not control emissions as well as newer equipment.
Effects of acid rains Ecological consequences of acid rain: - Acid rain damages and kills trees, fish and other wildlife. - It also affects human health. Aluminum (Al3+) in natural waters can cause Alzheimer disease – loss of short-term memory. - Acidity is not always the direct cause of damage. Acid rain can change soil and water. It dissolves nutrients and toxic heavy metals in the soil, and these are then washed into water of rivers and lakes. As a result of this, the soil lacks nutrients and toxic heavy metals in the soil. It can no longer continue to support trees; freshwater plant and animal life is very often sensitive to the presence of heavy metals. - Acid rain corrodes also building stone (limestone and marble), fabrics, and metallic constructions. 5. Sustainable strategies on ozone layer & acid rain problems. International cooperation Industries can help to prevent further damage to the ozone layer:
|
||||
|
Последнее изменение этой страницы: 2016-12-12; просмотров: 232; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.221.52.77 (0.008 с.) |

 Maximum Admissible Concentration (MAC) of pollutant is standard level of air pollutant below which there is virtually no harm to human health or environment. These standards are set to protect public health.
Maximum Admissible Concentration (MAC) of pollutant is standard level of air pollutant below which there is virtually no harm to human health or environment. These standards are set to protect public health.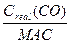 = 2
= 2