Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Electrochemical processes application ⇐ ПредыдущаяСтр 3 из 3
There are various extremely important electrochemical processes in both nature and industry, like the coating of objects with metals or metal oxides through electrodeposition and the detection of alcohol in drunken drivers through the redox reaction of ethanol. The generation of chemical energy through photosynthesis is inherently an electrochemical process, as is production of metals like aluminum and titanium from their ores. Certain diabetes blood sugar meters measure the amount of glucose in the blood through its redox potential. The action potentials that travel down neurons are based on electric current generated by the movement of sodium and potassium ions into and out of cells. Specialized cells in certain animals like the electric eel can generate electric currents powerful enough to disable much larger animals. Translate the text into Кazakh and Retelling the text. Electrolytic dissociation theory Electrolytic dissociation is decomposition of the electrolytes into the ions at the dissolution in water. In 1887 Swedish scientist Svante Arrhenius offered the theory of the electrolytic dissociation. According to the theory there are the following basic positions: 1). at dissolution in water the electrolytes decompose into positive and negative ions (dissociation). The word “ion” in translation from Greek means “strolling”. In a solution ions are disorderly move in different directions; 2). from electric current influence the ions have directional motion: positively charged ions to the cathode, negatively charged ions to the anode; 3). in all cases the process of the electrolytic dissociation is reversible. Together with decomposition of the molecules into ions (dissociation) also the ions joining process (association) is carried out:
Translate the text into Кazakh and Retelling the text. Types and properties of the solution There are 3 types of solutions according to the amount of dissolved substance: 1) Saturated solution is solution, in which at given temperature a substance is not dissolved. 2) Unsaturated solution is solution, in which at given temperature there is situated less amount of a dissolved substance, than in the saturated solution. 3) Re-saturated solution is solution, in which at given temperature in dissolved condition there is more substance amount, than in the saturated solution. Solution is described as “ concentrated”, if it consists of a large amount of solution and a small amount of solvent. A small amount of solute in a large amount of solvent results in a “dilute” solution. In dissolution process molecules of dissolved substance and solvent are not only mixed (diffused) between theselves, but also interact and form molecular compounds. In anhydrous solution these compounds are called solvates and in aqueous solution they are called hydrates. The solvates and hydrates are inconstant in many cases. After separation of dissolved substance they come off. Some hydrates are constant, for example: crystal-hydrate. Crystal-hydrate is solid substance which has water molecules. The water molecules are called crystal water. Translate the text into Кazakh and Retelling the text. Nuclear reactions The transformation process of one element into another element is called nuclear reaction. When we write the nuclear reaction equation, we must keep the equality of the particles charges and masses situated in the left and right sides. The phenomenon of transformation into another element at the decomposition of the elements nuclei are called natural radioactivity dissociation (all elements after uranium are predisposed to radioactive dissociation). At natural radioactivity atoms nuclei radiate from one of 1) 2)
3)
Translate the text into Кazakh and Retelling the text.
|
|||||
|
Последнее изменение этой страницы: 2016-06-26; просмотров: 63; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.219.22.169 (0.004 с.) |
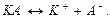
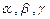 rays.
rays.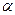 - helium nucleus
- helium nucleus 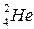 ;
;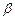 - high-speed stream of electrons;
- high-speed stream of electrons; - electrons stream;
- electrons stream;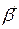 - positron stream.
- positron stream.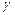 - short electromagnetic waves.
- short electromagnetic waves.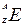
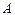 – mass number;
– mass number;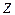 – atomic number.
– atomic number.