
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
The text hereafter deals with radiation chemistry. With what ideas and names is it associated for you? Complete the chart and suggest as many collocations as possible.Содержание книги
Поиск на нашем сайте
UNIT 8
RADIATION CHEMISTRY Radiation chemistry is the study of chemical changes resulting from the absorption of high-energy ionizing radiation. Such radiation includes alpha particles, electrons, gamma rays, fission fragments, protons, deuterons, helium nuclei, and heavier charged projectiles. X-rays are distinguished from gamma rays only as being extranuclear in origin. In absorbing materials of low and intermediate atomic weight such as aqueous systems and most biological systems, these radiations deposit energy in a largely indiscriminate manner, leaving behind a complex mixture of short-lived ions, free radicals, and electronically excited molecules. This contrasts with the absorption of visible and ultraviolet radiation, in which one or a few specific electronically excited species are formed. Radiation chemical change results from the further reaction of these intermediates.
TEXT A Pre-Reading Task The text hereafter deals with radiation chemistry. With what ideas and names is it associated for you? Complete the chart and suggest as many collocations as possible.
What applications of radioactivity can you name and in what spheres is it used more often?
RADIATION CHEMISTRY Radiation chemistry is the study of chemical changes resulting from the absorption of high-energy ionizing radiation, including alpha particles, electrons, gamma rays, fission fragments, protons, deuterons, helium nuclei, etc. In absorbing materials of low and intermediate atomic weight such as aqueous systems and most biological systems, such radiation deposits energy in a largely indiscriminate manner, leaving behind a complex mixture of short-lived ions, free radicals, and electronically excited molecules. Radiation-induced chemical changes result from reactions with these intermediates. Radiation chemistry is also used to study nuclear reactions such as fission and fusion. Some early evidence for nuclear fission was the formation of a short-lived radioisotope of barium which was isolated from neutron irradiated uranium. At that time, it was thought that this was a new radium isotope, as it was then standard radiochemical practice to use a barium sulphate carrier precipitate to assist in the isolation of radium. More recently, a combination of radiochemical methods and nuclear physics has been used to try to make new 'super heavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated. COMPREHENSION ASPECT Ex.1. Say whether the following statements are true or false. Correct the false ones. 1. Radiation chemistry is used to study nuclear reactions and chemical changes resulting from the absorption of high-energy ionizing radiation. 2. With all chemical elements, an isotope may be incorporated in a chemical compound, and thereafter, masses of this compound may be measured with a high precision. 3. The disintegration rate is completely unaffected by the chemical form of the isotope, and conversely, the property of radioactivity does not affect the chemical properties of the isotope. 4. Carbon-14 is constantly being created in the atmosphere by the interaction of cosmic rays with atmospheric nitrogen. 5. Accelerator mass spectrometry counts the amount of beta radiation emitted by decaying carbon-14 atoms in a sample, therefore it can be used with very small samples. 6. A larger dose of iodine-131 than that given to detect thyroid disease can’t be given to treat the disease, since the radioactive iodine accumulates in the thyroid and emits beta and gamma rays, which are considered to be very harmful for organism. 7. Radioisotopes called tracers are used by chemists and biochemists to determine the effects of using herbicides, pesticides, and fertilizers.
Ex.2. Match the words and expressions in the left-hand column with their definitions in the right hand column. They appear in the text you have just read.
Ex.3. Complete the following passage using the words from the box.
Traditional chemical reactions (1) … as a result of the interaction between valence electrons around an atom's nucleus. Nuclear reactions involve (2)... in particles in an atom's nucleus and thus (3)... a change in the atom itself. (4)... normal chemical reactions that form molecules, (5)... reactions result in the transmutation of one element into a different isotope or a different element altogether (remember that the (6) … of protons in an atom defines the element, so a change in (7) … results in a change in the atom). While many elements (8) … radioactive decay naturally, nuclear reactions can also be stimulated (9) ….
Ex.4. Divide the text into logical parts. Find and write out the main idea of each part. Highlight the key words that help you to generate your ideas. Write a three-sentence summary of the text using your key words, without referring back to the text. Then compare your summary with the rest of the class.
Ex.5. Split into groups or pairs and discuss the following questions. Make your own research, find out the necessary information. 1. What is the subject of study of radiation chemistry? What original erroneous conclusion was made concerning a short-lived radioisotope of barium which was isolated from neutron irradiated uranium and how was it corrected later? 2. What fundamental properties exhibited by all radioactive substances is the widespread use of isotopes based on? 3. Fill in the table dwelling on radioisotope applications.
GRAMMAR ASPECT THE PARTICIPLE
Note: Participle I expresses simultaneous action with the main verb. · Studying the properties of any substance, the chemist has to perform a number of experiments. · There are a number of different procedures being used in qualitative analysis.
Perfect participleexpresses the action preceding the action expressed by the main verb: · Having finished the experiment we must process the data. · Having been shown this chart we paid attention to the figures.
Participle II has the meaning of the passive voice. · The two substances investigated were homogeneous. · When cooled to the original temperature the substance becomes solid. NB! Pay attention to the translation of some special cases of Participle II. · The acid involved acted as a catalyst. Используемая кислота действовала в качестве катализатора. · The substance affected by a magnetic field must be a metal. Вещество, на которое воздействует магнитное поле, должно быть является металлом. · When (if) carried out carefully, this experiment can give reliable data. При аккуратном проведении этот эксперимент может дать достоверные данные.
Ex.1. Choose the correct form of the Participle. 1. ( Being, having been) very brittle antimony can be easily pulverized. 2. When (preparing, prepared) a substance on a commercial scale the method chosen must utilize inexpensive and readily available materials. 3. When (heating, heated) concentrated sulphuric acid reacts with metals. 4. Many ethers take up oxygen from the atmosphere, (forming, formed) peroxyethers. 5. Organometallic compounds are exceedingly reactive, (being hydrolyzed, having been hydrolyzed, hydrolyzed) vigorously by water. 6. Neutral water when (saturating, saturated) with O2 is a fairly good (oxidizing, oxidized) agent. 7. There are only few elements not (attacking, attacked) by oxygen. 8. Magnesium is not (attacking, attacked) by water despite favorable potential unless (amalgamating, amalgamated). 9.Carbon reduction (followed, following) roasting to oxides is used to prepare tin and lead fromtheir ores. 10. Once (starting, started) some reactions liberate heat and light. 11. Oxygen as ordinarily (obtained, obtaining) is widely used in laboratory practice. 12. The steam (liberating, liberated) condensed as the temperature fell. 13. The (discussed, discussing) compounds are characteristic of the transition metals. 14. The electron volt (e.v.) is the kinetic energy (being acquired, acquiring) by an electron when it passes through potential difference of 1 volt. 15. (Indicating, indicated) the proportion of hydrogen and oxygen present in sulphuric acid the formula H2SO4 is used. 16. Tinand lead are prepared from their ores by (roasted, roasting) to oxides (followed, following) by carbon reduction.
Ex.2. Open the brackets translating the Russian words into English. 1. Oxygen can be converted to a liquid (путем кипения) at − 183°C. 2. Oxides of bismuth are basic, (образующие) salts such as bismuth chloride. 3. The iodine ions are easily oxidized, (изменяясь) to free iodine by chlorine, oxygen and many other oxidizing agents. 4. (Охладив) the concentrated solution of naphthalene in hexane we obtained white precipitate of pure naphthalene. 5. (После пропускания) through a hot tube hydrogen arsenide deposited arsenic in form of metallic film. 6. The salts (образованные) by hydrochloric acid are called chlorides. 7. Copper tarnishes when (выставлена) to air. 8. Solutions (содержащие) much of the solute and little of the solvent are called strong or concentrated solutions. 9. Any element (при соединении) with oxygen forms oxide. 10. (Получив) the necessary data on the density of the liquid we could begin the determination of the index of refraction. 11. (После охлаждения) to a very low temperature many substances acquire quite new properties. 12. (При нагревании) concentrated sulphuric acid reacts with metals. 13. Hydrogen burns readily in air or in oxygen, and product (образованный) is water. 14. Radioactivity is the property (не поддающееся влиянию) by any known catalyst. 15. Inert gases are the substances (не поддающиеся воздействию) by oxygen. Ex.3. Translate the following sentences, pay attention to the forms and functions of the Participles. 1. Bond formation involving elements toward the middle of the periodic table occurs by the process of electron sharing. 2. The calculation of hydrogen ion concentration in a solution containing a weak electrolyte is a difficult one for the average student first facing this problem. 3. Elements composed of atoms containing only one or two valence electrons usually form positive ions. 4. We are surrounded by naturally-occurring radioactive elements in the soil and stones. 5. The amount of energy deposited in a particular mass of human tissue is called the absorbed dose. 6. The equivalent dose is expressed in a unit called the Sievert. 7. Radiation doses normally encountered are expressed in millisievert or microsievert. 8. The total of weighted equivalent doses is a quantity called the effective dose. 9. Radioisotopes decay at specific rates called half-lives. Ex.4. Translate the following sentences into English. 1. Вода, используемая в паровых котлах, должна быть свободна от веществ, вызывающих коррозию. 2. Работа, проведенная учеными, имела большое значение. 3. Машины, производящие эти товары, должны быть обновлены. 4. Этот вид излучения состоит из тока положительно заряженных частиц. 5. Производя новые виды материалов, следует особо внимательно относиться к их качеству. 6. Завод по производству этих товаров был построен в прошлом году. 7. Открыв радиоактивность, учёные подняли ряд новых вопросов. 8. Вещество, исследуемое нами, может быть использовано и в этом эксперименте. 9. Очистив воду от веществ, вызывающих коррозию, мы можем использовать её в паровых котлах. 10. Волокна, исследованные ими, были достаточно прочны. 11. Метод, о котором идет речь, зависит от ряда факторов, описанных выше. 12. Энергия, образующаяся путем расщепления ядра атома, называется ядерной или атомной энергией. 13. В основном атомы содержат незаряженные частицы, называемые нейтронами. 14. При нагревании молекулы жидкости движутся быстрее. 15. Изучив все свойства новой воды, они смогли понять тайну серебристых облаков. 16. Растворенные материалы могут быть растворимыми твердыми веществами, жидкостями или газами.
TEXT B RADIATION PROTECTION Read the text and make notes to identify its main points.Then consider the following questions: TEXT C NUCLEAR REACTORS Before reading the text: Thermal Reactors Currently the majority of nuclear power plants in the world are water-moderated, thermal reactors. They are categorized as either light water or heavy water reactors. Light water reactors use purified natural water ( None of the water flowing through the reactor core leaves the containment structure. In a BWR, the water flowing through the core is converted directly to steam and leaves the containment structure to drive the turbines. Light water reactors use low enriched uranium as fuel. Enriched fuel is required because natural water absorbs some of the neutrons, reducing the number of nuclear fissions. All of the 103 nuclear power plants in the United States are light water reactors; 69 are PWRs and 34 are BWRs. Fast Neutron Reactors In contrast to thermal reactors, the neutrons in a fast neutron reactor (or fast breeder reactor, FBR) are not slowed by the presence of a moderator. The coolant, usually a liquid sodium or lead, is a substance that does not slow or absorb neutrons. It also has excellent heat transfer properties, which allow the reactor to be operated at lower pressures and higher temperatures than thermal reactors. An FBR is configured and operated to produce more fuel than it consumes. Fast neutrons are readily absorbed by fertile uranium-238, which then can undergo successive beta emissions to become fissile Pu-239. Thorium-232 is another fertile isotope that can absorb neutrons and produce fissile uranium-233 by beta emissions. These fissile isotopes can be reprocessed for nuclear reactor fuel or weapons. Because fast neutrons are not as efficient in producing fission as slow ones, FBRs require uranium oxide containing 20% U-235, plutonium oxide, or a mixture of these oxides, known as MOX, as fuel. Originally FBRs were thought to be a means of extending global uranium resources by producing fissile Pu-239 or U-233 as reactor fuel. However, problems with reactor operations and material components combined with the discovery of new uranium deposits mean that FRBs are not economically competitive with existing thermal reactors. FBR research has produced technical advances but the limiting factor continues to be the price of FBR-produced reactor fuel versus the cost of uranium fuel. FBRs are more complex than other types of reactors and also raise concerns about the proliferation of plutonium for use in nuclear weapons.
UNIT 8
RADIATION CHEMISTRY Radiation chemistry is the study of chemical changes resulting from the absorption of high-energy ionizing radiation. Such radiation includes alpha particles, electrons, gamma rays, fission fragments, protons, deuterons, helium nuclei, and heavier charged projectiles. X-rays are distinguished from gamma rays only as being extranuclear in origin. In absorbing materials of low and intermediate atomic weight such as aqueous systems and most biological systems, these radiations deposit energy in a largely indiscriminate manner, leaving behind a complex mixture of short-lived ions, free radicals, and electronically excited molecules. This contrasts with the absorption of visible and ultraviolet radiation, in which one or a few specific electronically excited species are formed. Radiation chemical change results from the further reaction of these intermediates.
TEXT A Pre-Reading Task The text hereafter deals with radiation chemistry. With what ideas and names is it associated for you? Complete the chart and suggest as many collocations as possible.
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2019-04-30; просмотров: 155; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.15.31.27 (0.009 с.) |



 ) as the coolant/moderator, while heavy water reactors employ heavy water, deuterium oxide (
) as the coolant/moderator, while heavy water reactors employ heavy water, deuterium oxide (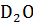 ). In light water reactors, the water is either pressured to keep it in superheated form (in a pressurized water reactor, PWR) or allowed to vaporize, forming a mixture of water and steam (in a boiling water reactor, BWR). In a PWR, superheated water flowing through tubes in the reactor core transfers the heat generated by fission to a heat exchanger, which produces steam in a secondary loop to generate electricity.
). In light water reactors, the water is either pressured to keep it in superheated form (in a pressurized water reactor, PWR) or allowed to vaporize, forming a mixture of water and steam (in a boiling water reactor, BWR). In a PWR, superheated water flowing through tubes in the reactor core transfers the heat generated by fission to a heat exchanger, which produces steam in a secondary loop to generate electricity.


