
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Warning: not for treatment of obesity or for weight lossСодержание книги
Поиск на нашем сайте
INDICATIONS AND USAGE CYTOMEL is an L-triiodothyronine (T3) indicated for:
Limitations of Use: - Not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients. (1) - Not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS Tablets: 5 mcg, 25 mcg, 50 mcg (3) CONTRAINDICATIONS Uncorrected adrenal cortical insufficiency (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS Most common adverse reactions for CYTOMEL are primarily those of hyperthyroidism due to therapeutic overdosage: arrhythmias, myocardial infarction, dyspnea, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to CYTOMEL (7) USE IN SPECIFIC POPULATIONS Pregnancy may require the use of higher doses of thyroid hormone (2.2, 8.1) See 17 for PATIENT COUNSELING INFORMATION. Revised: 12/2018 |
INDICATIONS AND USAGE
Hypothyroidism
CYTOMEL is indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Thyroid Suppression Test
CYTOMEL is indicated as a diagnostic agent in suppression tests to differentiate suspected mild hyperthyroidism or thyroid gland autonomy.
Limitations of Use
- CYTOMEL is not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients as there are no clinical benefits and overtreatment with CYTOMEL may induce hyperthyroidism [see Warnings and Precautions (5.4)].
- CYTOMEL is not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets (round, white to off-white) available as follows:
- 5 mcg: debossed with KPI on one side and 115 on the other side
- 25 mcg: scored on one side and debossed with KPI and 116 on the other side
- 50 mcg: scored on one side and debossed with KPI and 117 on the other side
CONTRAINDICATIONS
CYTOMEL is contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
WARNINGS AND PRECAUTIONS
Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism, and may result in unpredictable absorption of thyroid hormone from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
ADVERSE REACTIONS
Adverse reactions associated with CYTOMEL therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5.4) and Overdosage (10)]. They include the following:
General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
Musculoskeletal: tremors, muscle weakness and cramps
Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
Respiratory: dyspnea
Gastrointestinal: diarrhea, vomiting, abdominal cramps, elevations in liver function tests
Dermatologic: hair loss, flushing
Endocrine: decreased bone mineral density
Reproductive: menstrual irregularities, impaired fertility
Adverse Reactions in Pediatric Patients
Pseudotumor cerebri and slipped capital femoral epiphysis have been reported in pediatric patients receiving thyroid replacement therapy. Overtreatment may result in craniosynostosis in infants and premature closure of the epiphyses in pediatric patients with resultant compromised adult height.
Hypersensitivity Reactions
Hypersensitivity reactions to inactive ingredients have occurred in patients treated with thyroid hormone products. These include urticaria, pruritus, skin rash, flushing, angioedema, various gastrointestinal symptoms (abdominal pain, nausea, vomiting and diarrhea), fever, arthralgia, serum sickness and wheezing.
DRUG INTERACTIONS
Antidiabetic Therapy
Addition of CYTOMEL therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control, especially when CYTOMEL is started, changed, or discontinued [see Warnings and Precautions (5.5)].
Oral Anticoagulants
CYTOMEL increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the CYTOMEL dose is increased. Closely monitor coagulation tests to permit appropriate and timely dosage adjustments.
Digitalis Glycosides
CYTOMEL may reduce the therapeutic effects of digitalis glycosides. Serum digitalis glycoside levels may be decreased when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and CYTOMEL may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and central nervous system stimulation. CYTOMEL may accelerate the onset of action of tricyclics. Administration of sertraline in patients stabilized on CYTOMEL may result in increased CYTOMEL requirements.
Ketamine
Concurrent use of ketamine and CYTOMEL may produce marked hypertension and tachycardia. Closely monitor blood pressure and heart rate in these patients.
Sympathomimetics
Concurrent use of sympathomimetics and CYTOMEL may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
Tyrosine-Kinase Inhibitors
Concurrent use of tyrosine-kinase inhibitors such as imatinib may cause hypothyroidism. Closely monitor TSH levels in such patients.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Experience with liothyronine use in pregnant women, including data from post-marketing studies, have not reported increased rates of major birth defects or miscarriages (see Data). There are risks to the mother and fetus associated with untreated hypothyroidism in pregnancy. Since TSH levels may increase during pregnancy, TSH should be monitored and CYTOMEL dosage adjusted during pregnancy (see Clinical Considerations). There are no animal studies conducted with liothyronine during pregnancy. CYTOMEL should not be discontinued during pregnancy and hypothyroidism diagnosed during pregnancy should be promptly treated.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Maternal hypothyroidism during pregnancy is associated with a higher rate of complications, including spontaneous abortion, gestational hypertension, pre-eclampsia, stillbirth, and premature delivery. Untreated maternal hypothyroidism may have an adverse effect on fetal neurocognitive development.
Dose adjustments during pregnancy and the postpartum period
Pregnancy may increase CYTOMEL requirements. Serum TSH levels should be monitored and the CYTOMEL dosage adjusted during pregnancy. Since postpartum TSH levels are similar to preconception values, the CYTOMEL dosage should return to the pre-pregnancy dose immediately after delivery [see Dosage and Administration (2.3)].
Data
Human Data
Liothyronine is approved for use as a replacement therapy for hypothyroidism. Data from post-marketing studies have not reported increased rates of fetal malformations, miscarriages, or other adverse maternal or fetal outcomes associated with liothyronine use in pregnant women.
Lactation
Risk Summary
Limited published studies report that liothyronine is present in human milk. However, there is insufficient information to determine the effects of liothyronine on the breastfed infant and no available information on the effects of liothyronine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CYTOMEL and any potential adverse effects on the breastfed infant from CYTOMEL or from the underlying maternal condition.
Pediatric Use
The initial dose of CYTOMEL varies with age and body weight. Dosing adjustments are based on an assessment of the individual patient's clinical and laboratory parameters [see Dosage and Administration (2.3, 2.4)].
In pediatric patients in whom a diagnosis of permanent hypothyroidism has not been established, discontinue thyroid hormone for a trial period, but only after the child is at least 3 years of age. Obtain serum TSH, T4, and T3 levels at the end of the trial period, and use laboratory test results and clinical assessments to guide diagnosis and treatment, if warranted [see Dosage and Administration (2.6)].
Congenital Hypothyroidism [see Dosage and Administration (2.2, 2.6)]
Rapid restoration of normal serum T4 concentrations is essential for preventing the adverse effects of congenital hypothyroidism on intellectual development as well as on overall physical growth and maturation. Therefore, initiate thyroid hormone immediately upon diagnosis. Thyroid hormone is generally continued for life in these patients.
Closely monitor infants during the first 2 weeks of thyroid hormone therapy for cardiac overload, arrhythmias, and aspiration from avid suckling.
Closely monitor patients to avoid undertreatment or overtreatment. Undertreatment may have deleterious effects on intellectual development and linear growth. Overtreatment is associated with craniosynostosis in infants, may adversely affect the tempo of brain maturation, and may accelerate the bone age and result in premature epiphyseal closure and compromised adult stature [see Dosage and Administration (2.6) and Adverse Reactions (6)].
Acquired Hypothyroidism in Pediatric Patients
Closely monitor patients to avoid undertreatment and overtreatment. Undertreatment may result in poor school performance due to impaired concentration and slowed mentation and in reduced adult height. Overtreatment may accelerate the bone age and result in premature epiphyseal closure and compromised adult stature.
Treated children may manifest a period of catch-up growth, which may be adequate in some cases to normalize adult height. In children with severe or prolonged hypothyroidism, catch-up growth may not be adequate to normalize adult height [see Adverse Reactions (6)].
Geriatric Use
Because of the increased prevalence of cardiovascular disease among the elderly, initiate CYTOMEL at less than the full replacement dose [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)]. Atrial arrhythmias can occur in elderly patients. Atrial fibrillation is the most common of the arrhythmias observed with thyroid hormone overtreatment in the elderly.
OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5.4) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, seizure, shock, coma, and death have been reported. Symptoms may not necessarily be evident or may not appear until several days after ingestion.
Reduce the CYTOMEL dose or temporarily discontinued if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient's medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
DESCRIPTION
CYTOMEL tablets contain the active ingredient, liothyronine (L-triiodothyronine or LT3), a synthetic form of a thyroid hormone liothyronine in sodium salt form. It is chemically designated as L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-, monosodium salt. The molecular formula, molecular weight and structural formula of liothyronine sodium are given below.
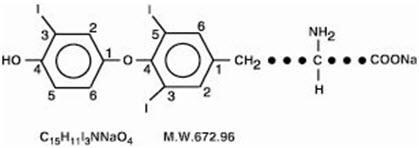
CYTOMEL tablets contain liothyronine sodium equivalent to liothyronine in 5 mcg, 25 mcg, and 50 mcg. Inactive ingredients consist of calcium sulfate, corn starch, gelatin, stearic acid, sucrose and talc.
CLINICAL PHARMACOLOGY
Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
Pharmacodynamics
The onset of activity of liothyronine sodium occurs within a few hours. Maximum pharmacologic response occurs within 2 or 3 days.
Pharmacokinetics
Absorption
T3 is almost totally absorbed, 95 percent in 4 hours. The hormones contained in the natural preparations are absorbed in a manner similar to the synthetic hormones.
Distribution
Liothyronine sodium (T3) is not firmly bound to serum protein. The higher affinity of levothyroxine (T4) for both thyroid-binding globulin and thyroid-binding prealbumin as compared to triiodothyronine (T3) partially explains the higher serum levels and longer half-life of the former hormone. Both protein-bound hormones exist in reverse equilibrium with minute amounts of free hormone, the latter accounting for the metabolic activity.
Elimination
Metabolism
The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately 80% of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3. T3 is further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Excretion
Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged and is eliminated in the feces. The biological half-life is about 2–1/2 days.
NONCLINICAL TOXICOLOGY
INDICATIONS AND USAGE
CYTOMEL is an L-triiodothyronine (T3) indicated for:
- Hypothyroidism: As replacement in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism (1.1)
- Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) Suppression: As an adjunct to surgery and radioiodine therapy in the management of well-differentiated thyroid cancer (1.2)
- Thyroid Suppression Test: As a diagnostic agent in suppression tests to differentiate suspected mild hyperthyroidism or thyroid gland autonomy (1.3)
Limitations of Use:
-
Not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients. (1)
-
Not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis. (1)
DOSAGE AND ADMINISTRATION
- Administer CYTOMEL orally once daily and individual dosage according to patient response and laboratory findings (2.1)
- See full prescribing information for recommended dosage for hypothyroidism (2.2) TSH suppression in well-differentiated thyroid cancer (2.3) and for thyroid suppression test (2.4)
- When switching a patient to CYTOMEL, discontinue levothyroxine therapy and initiate CYTOMEL at a low dosage. Gradually increase the dose according to the patient's response (2.5)
- Adequacy of therapy determined with periodic monitoring of TSH and T3 levels as well as clinical status (2.6)
DOSAGE FORMS AND STRENGTHS
Tablets: 5 mcg, 25 mcg, 50 mcg (3)
CONTRAINDICATIONS
Uncorrected adrenal cortical insufficiency (4)
WARNINGS AND PRECAUTIONS
- Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate CYTOMEL at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation (2.3, 5.1, 8.5)
- Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.2)
- Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of CYTOMEL treatment (5.3)
- Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism. (5.4)
- Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy (5.5)
- Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose (5.6)
ADVERSE REACTIONS
Most common adverse reactions for CYTOMEL are primarily those of hyperthyroidism due to therapeutic overdosage: arrhythmias, myocardial infarction, dyspnea, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to CYTOMEL (7)
USE IN SPECIFIC POPULATIONS
Pregnancy may require the use of higher doses of thyroid hormone (2.2, 8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
- Thyroid hormones, including CYTOMEL, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), and Overdosage (10)].
INDICATIONS AND USAGE
Hypothyroidism
CYTOMEL is indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
|
| Поделиться: |




