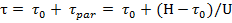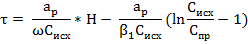Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Define the following terms: The coefficient of protective action (at saturation of the ion exchanger)
Full time (t) work the resin layer height H is equal to the sum of the time of formation of the front concentrations (t0) and parallel transfer time concentrations Front (tpar):
where U - speed of the concentration front, its value is determined by the equilibrium capacity of the resin, the solution flow rate and initial concentration of the substance in the solution recovered. The reciprocal of the speed of parallel movement of front K = 1 / U is called the coefficient of the protective action and is the time during which the resin layer is 1 m tall fully saturated with recoverable material. To determine the protective effect of the resin layer to find the height of the working layer of the resin H0. When the external diffusion kinetics of the rate of change recovered substance concentration in the solution height of the working layer of the resin described by the equation: dC / dt = -β1(C – Cпр) where b1 is kinetic coefficient of external diffusion, Cпр - the concentration of extractable substances in solution at breakthrough is close to 0. Thus, while the resin layer of the protective effect found from Eq
Causes of time loss of the protective effect of the following: 1) ion exchange equilibrium is not instantaneous, of the extracted substance, failing to satisfy the first layer, is absorbed in the next; 2) there is leakage channel solution associated with the uneven stacking the resin grains; 3) "wall" effect is faster progress flow at the walls.
Combine options for process “sorption” – “desorption”. Draw your own version of the technological scheme of anion-exchange extraction of uranium from sulfuric acid solutions. Acid leaching method is usually applied in Kazakhstan. In this task, I must offer its own version of the sorption and desorption of uranium solutions. Therefore, it may be suggested that: In the case of high content of carbonates in the Earth's rocks is necessary to use the carbonate leaching method. Using of acid method is not productive, because sulfuric acid will neutralize the carbonate rocks of the earth and uranium will not be solved. Carbonates form anionic complexes of uranium. Pumping wells transport the solution to the sorption column. These columns are full of anion exchange resin. Passing through the resin uranium anions are adsorbed and remain on the resin. At the outlet of the column is obtained without solution of uranium. After saturation of the resin, the resin is distilled in the desorption column. Desorption can be carried out by liquid extraction. Usually, the problem of the extraction method of stripping is the complexity of cleaning the grain from the anion exchanger extractants. The result is that repeated application difficult. In the case of the carbonate method, this problem is easily removable, as are carbonate ions surfactants. That is dissolved and removed from the surface of the grains extractants ion exchangers. This method can be proposed as an alternative method, the acid leaching of uranium
|
||||
|
Последнее изменение этой страницы: 2017-02-17; просмотров: 46; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.144.98.13 (0.004 с.) |