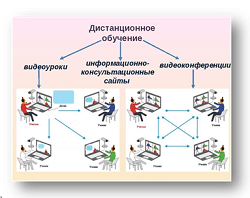
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь FAQ Написать работу КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Chemical and mineralogical composition aliteСодержание книги
Поиск на нашем сайте Portland cement clinker includes several artificial minerals formed during firing. Approximate content of minerals in the four main clinker of Portland cement '(% by weight): alite 3CaO-SiO2 (C3S) - 40... 65, belite 2CaO-SiO2 (C2S) - 15... 40, celite CJSIC-A12O3 (C3A) - 5... 15 celite 4SaO-Al2O3-Fe2O3 (C4AF) - 10... 20. cement clinker microscopic examination showed that it is dominated by alite and belite crystals, which is located between the intermediate substance consisting of calcium aluminates and alyumoferritov in crystalline form, as well as vitreous glass and left in a free state CaO and MgO. Tricalcium silicate (alite) - the main mineral of cement clinker - has a high activity in the reaction with water, especially in the initial period of time (the quantity of heat to 3 days up to about 2 / s of heat at full hydration). Alit quickly hardens and gains high strength. Alyth - the main mineral clinker hardens quickly and practically determines the rate of hardening and increase the strength of Portland cement. He is a solid solution of tricalcium silicate and a small amount (2... 4%), other impurities, which can significantly affect the structure and properties of Portland cement.
Chemical and mineralogical composition of belite Portland cement clinker includes several artificial minerals formed during firing. Approximate content of minerals in the four main clinker of Portland cement '(% by weight): alite 3CaO-SiO2 (C3S) - 40... 65, belite 2CaO-SiO2 (C2S) - 15... 40, celite CJSIC-A12O3 (C3A) - 5... 15 celite 4SaO-Al2O3-Fe2O3 (C4AF) - 10... 20. cement clinker microscopic examination showed that it is dominated by alite and belite crystals, which is located between the intermediate substance consisting of calcium aluminates and alyumoferritov in crystalline form, as well as vitreous glass and left in a free state CaO and MgO. Dicalcium silicate (belite) is considerably less active than alite. Heat at full hydration of belite is approximately 2 times smaller than that of alite, and d 3 is about 10% of the total heat release during hydration. Belita Hardening occurs slowly. For a month's time the product has hardened its relatively low strength, but prolonged hardening (several years) in favorable conditions (at positive temperature and humid environment), its strength has been steadily increasing. Belit - second in importance and content of clinker silicate minerals, slowly hardens and attains a high strength at prolonged curing. Belite clinker is a solid solution of dicalcium silicate and a small amount (1%... 3) other. Contaminants. Due to the fact that the belite clinker slow cooling becomes astringent properties, this phenomenon is prevented by rapid cooling of the clinker. Phase aluminate Aluminate phase content is 5-10% for most normal cement clinkers. It tricalcium aluminate 3SaO * Al2O3, significantly modified the composition and sometimes the structure, due to foreign ions, especially Si4 +, Fe3 +, Na + and K +. Aluminate phase reacts rapidly with water and may cause undesirably rapid setting, if no seizure supervising added reagent commonly gypsum. Aluminate phase is usually credited with the formula trehkaltsie Vågå-aluminate (S3L), although the amount determined by microscopic and X-ray methods, usually less than this estimate. This is because a certain amount of alumina compounds dissolved in the silicate clinker. Alumina is included in the composition of the aluminate phases of Portland cement. Elevated amounts accelerate its setting time and a decrease in the durability of stone in the mineralized waters. When hydration of granulated blast slag aluminate phase turns into hydrogarnet similar in composition and properties from the hexahydrate calcium hydroaluminates or in gidroalyuminatnye siliceous phases of variable composition. However, it was found a discrepancy in the definition phase of the aluminate, which causes the need to improve the clinker composition assessment methods. Young showed that C3A strongly adsorbs surfactants from solution and therefore with a considerable content of aluminate phases to mitigate hydration needs a high percentage lignosulfate. When calculating the mineralogical composition take that into the clinker contains only pure minerals C3S, C2S, C3A, C4AF; aluminate C3A phase is represented; sulfates - sul calcium sulfonate. It is believed that no vitreous phase. The advantage of sodium nitrite, as well as other, nitrite salts introduced into the concrete mixture, consists in the fact that the nitrite ion with the aluminate phases unreacted cement and hydroaluminates cement stone calcium interacts with a slower rate and leads to a sparingly soluble double salt hydrate: gidrshitrialyumI Nat calcium with less complete than the chloride ion.
Phase Ferrite Alyumoferritnaya phase ferritic phase (CaAlFe) is 5-15% of normal cement clinker. It - chetyrёhkaltsievy alumina ferrite 4CaO * Al2O3 * Fe2O3, the composition of which varies considerably when changing Al / Fe ratio in the structure and placement of foreign ions. The rate at which the ferrite phase is reacted with water, may vary somewhat due to differences in the composition or other characteristics, but usually, it is high in the initial period and is intermediate between the speed of alite and belite at a later date. The clinker is typically present in small amounts of several other phases such as alkali sulfates and calcium oxide.
Conditions of synthesis of silicates, aluminates and ferrites of calcium that make up clinker, as well as a number of intermediate compounds are important in the chemistry of cement. A significant role is played by the thermodynamic analysis in their study. As for the hydration reactions alyumoferrita calcium and iron, the complete lack of thermochemical data for calcium gidroferritov does not allow currently consider their thermodynamics. With further increase in temperature calcium ferrite lines intensity decreases and begins to appear 2CaO - AbOa-SiCfe, which at temperatures of 1100 - 1200 C becomes the main phase. The portland cement are silicates, aluminates and calcium ferrites formed in roasting raw Oomes. The resulting, after firing the raw mix product - clinker - is crushed and finely ground. A joint lomola clinker and additives yielding the final product - Portland cement. Clinker minerals (silicates, calcium aluminates and ferrites) by reacting with water undergo hydrolysis and the salts formed by a strong base and a weak acid. Hydrolysis of Tricalcium hydroaluminate formed and calcium ferrites. Clinker minerals (silicates, calcium aluminates and ferrites) by reacting with water undergo hydrolysis and the salts formed by a strong base and a weak acid. Locally on the boundaries between the crystals of magnetite and calcium ferrite observed globular discharge grayish-bluish crystals of potassium ferrite. This phase is anisotropic and hygroscopic, at high magnification, and especially in the study of immersion oil has a reddish-brown internal reflections. It has previously been shown that the catalysts prepared by standard methods (fuse resistance in melting furnaces and oxygen), potassium ferrites formed by introducing potassium hydroxide over 0.65 wt. Our research found that catalysts prepared at high temperatures, this phase is observed only in making potassium hydroxide over 2 wt. Based on these data it can be assumed that part of the potassium hydroxide dissolves magnetite lattice to form solid solutions isomorphous substitution, the remainder goes into a glassy phase. Formed during firing lime silicates, aluminates and calcium ferrites according to its quantitative content may more or less influence on the physico-chemical properties of lime. In pure limestone and sandy clay content of impurities is expressed in small amounts. Therefore bystrogasya formed schiesya-lime due to its high content of free calcium oxide. However, they can always contain some substances that are holders gidravlichnosti properties. These substances include silicates, aluminates and ferrites of calcium, formed due to the presence of limestone in the fuel ash and calcium oxide impurities. At a low content of these formations in the hydraulic properties of lime binder did not show up. Hydraulic hardening due to the presence of silicates, aluminates and ferrites of calcium that form in contact with water hydrosilicates, hydroaluminates and calcium gidroferrity. Hydraulic hardening due to the presence of silicates, aluminates and ferrites of calcium that form in contact with water hydrosilicates, gkdroalyuminaty and calcium gkdroferrity. With increasing content of silicates, aluminates and calcium ferrites conditions hardening hydraulic lime close to the conditions of curing romantsementa, and with increasing amounts of calcium hydroxide - to the conditions of an air hardening lime. The smaller the product would be fired in silicates, aluminates and calcium ferrites, so rapid and complete is extinguished lime and relatively more malleable dough is obtained.
Other clinker phases alite It is the most important part of all conventional cement clinkers; its content is 50-70%. It trёhkaltsievy silicate 3SaO * SiO2 (abbreviated C3S), the composition and structure of which are modified due to placement in the lattice of foreign ions, especially of Mg2 +, Al3 + and Fe3 +. Alite relatively quickly reacts with water and normal phases of all cements plays the most important role in the development of strength; daily for 28 strength contribution of this phase is particularly important. Belit Content for normal belite cement clinker is 15-30%. This dicalcium silicate 2SaO * SiO2 (C2S), modified by introducing into the structure of the foreign ions and usually completely or predominantly present in a β-modification. Belit slowly reacts with water, thus affecting the strength weak during the first 28 days, but significantly increases the strength at a later date. A year later, the strength of pure alite and belite net under comparable conditions are about the same. aluminate phase Aluminate phase content is 5-10% for most normal cement clinkers. It tricalcium aluminate 3SaO * Al2O3, significantly modified the composition and sometimes the structure, due to foreign ions, especially Si4 +, Fe3 +, Na + and K +. Aluminate phase reacts rapidly with water and may cause undesirably rapid setting, if no seizure supervising added reagent commonly gypsum. Alyumoferritnaya phase Ferrite phase (CaAlFe) 5-15% of a conventional cement clinker. It - chetyrёhkaltsievy alumina ferrite 4CaO * Al2O3 * Fe2O3, the composition of which varies considerably when changing Al / Fe ratio in the structure and placement of foreign ions. The rate at which the ferrite phase is reacted with water, may vary somewhat due to differences in the composition or other characteristics, but usually, it is high in the initial period and is intermediate between the speed of alite and belite at a later date. The clinker is typically present in small amounts of several other phases such as alkali sulfates and calcium oxide. In Portland cement clinker is often 1-2% free CaO (free lime). The reasons may be: lack of training materials (roughly chopped or non-homogeneous raw meal), lack of temperature and firing time, resulting CaOnepolnostyu associated with other oxides, too slow cooling, when the partial decomposition of C3S and C3A, or too high saturation coefficient KH> 0,96-0,98). The polished sections of clinker free lime in the etching is visible in the form of colored particles from turquoise to blue. Often one can observe that the free lime occurs in the nests (Figure 1.2). This is due in most cases to poor homogeneity of the raw meal. Free lime is undesirable because it may cause (for example, in amounts of> 2.5%) non-uniform change in volume of cement, hardened cement stone can expand and cause cracks. Lime cracks occur due to increased volumes of approximately 2-fold when hydrated CaO with conversion to Ca (OH) 2. Saturated MgO clinker may contain free MgO - periclase. Since approximately 2,0-2,5% MgO as well as other ions can be introduced into the clinker phases, part of MgO can remain free and form periclase. The standardized cement according to EN 197 German industrial standard can contain a maximum of 5,0% MgO, a maximum of 2.5-3.0% of free periclase. The content of MgO, which binds to the other phases, depending on the chemical composition of clinker and its manufacturing conditions. The polished sections of MgO clinker in most cases occur in the hexagonal form. It is necessary to distinguish between emerging pink colored crystals of periclase from tricalcium silicate. Along with the different colors by etching it detects smaller size crystals, common jacks. They are somewhat uplifted and polished areas are extracted from the clinker as MgO has a higher hardness than the other phases of the clinker. Periclase is undesirable, since a higher content of hazardous magnesium (similar free lime) may occur. Harmful magnesia insidious, since the destruction of concrete products may appear only after many years. The finely crystalline and evenly distributed periclase expansion phenomenon is significantly less than in the coarse MgO or "nests" MgO. It is also typical for a free free lime.
|
||
|
Последнее изменение этой страницы: 2017-02-05; просмотров: 710; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 216.73.216.214 (0.009 с.) |