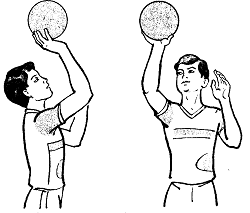
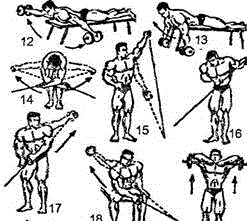
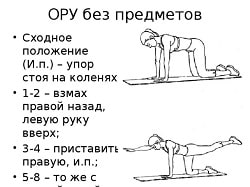
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Emulsion stability depends of ⇐ ПредыдущаяСтр 6 из 6
• 1. physical-chemical properties, • 2. colloidal particles size, • 3. temperature, • 4. intensify of blending, • 5. density and viscosity of oil.
• Steps for emulsion breaking • 1. Destruction of protecting layer • 2. Closing in the droplets • 3. Coagulation • 4. Sedimentation
• 1. mechanical
• 2. chemical
• 3. electric
Mechanical methods • Settling - heating under 120-160°С under pressure 8-15 atm within 2-3 hours
• Separation occurs because of preservative layer begin dissolve in crude oil at high temperature. • Centrifugation • Filtration
• Several methods are used to break water-oil emulsions: • Demulsifiers – They may decreasing and cancel the electrostatic forces of repulsion reacting between the water droplets. They also modify the wettability of the solid micro-particles absorbed at the interface or form some inverse emulsion oil-in-water, thus destabilizing the water-in-oil emulsion. • Agitation – Mixing increases the collision number between particles and their coalescence. In practice, the mixing is natural during the flow of the emulsion in surface equipment.
Requirements to crude oil
• Before transportation: • - water content W water<0,5%; • - salts content Р salt<200 mg/L.
• Before rectification: • - water content W water<0,05%; • - salts content Р salts<20 mg/L.
• Salts could hydrolyze and as result they can form a hydrochloric acid.
• Sand, Silts, Salt deposit and Foul Heat Exchangers
• Water Heat of Vaporization reduces crude Pre-Heat capacity
• Sodium, Arsenic and Other Metals can poison Catalysts
• Potassium, Calcium, Magnesium Chlorides and Bicarbonates
• CaCl2 is hydrolyzed about 10% • MgCl2 - - 90%
• М gCl 2 + Н2О = М gОН Cl + Н Cl • CaCl2 + H2O = CaOHCl + HCl
• Sulfur corrosion • Fe + H2S ® FeS + H2 • FeS + 2HCl ® FeCl2 + H2S Oil pretreatment: • 1. Stabilization and separation
• 2. Desalting
• 3. Dewatering (dehydration or drying)
Pretreatment of Crude Oil • Gas separators - for removing of dissolving gases from crude oil
• At the same time the separation of crude oil from mechanical impurities and the majority of water are occurred
Electrostatic Desalting • Polar water molecules are replaced with a strong electrostatic charge which drives the separation water from oil
• Electrical desalting is the application of high-voltage electrostatic charges to concentrate suspended water globules in the bottom of the settling tank.
Atmospheric Rectification
Heat and mass exchange process for separation of liquid into its components by their boiling points using the countercurrent multiply contact between vapor and liquid
• Reflux (liquid) –condensed overhead liquid which returns to the upper part of column for better separation of products
• Reflux (steam) – water steam which puts into bottom of column for better boiling Refux (steam) Reflux (steam) – water vapor is added into bottom of the column for decreasing of partial pressure of HC that decreases their boiling point
Due to reflux water vapor are intensively mixed with liquid hydrocarbons that promote the evaporation of low boiling HC Trays Towers • Main principle
• Continues contact between vapor and liquid and mutual concentration of definite hydrocarbons on the definite tray due to countercurrent flow
Packed column • Packing materials are used in PC instead of trays as in the TC
• The main property of the PC is the specific surface area which determines the efficiency of the packing • Packing materials are the different geometric forms from different materials. • They have under 70 mm diameters and the ratio of surface area to the volume is above 500 times • Differently shaped packing materials have different surface areas and void space between the packing. Both of these factors affect packing performance. • Atmospheric rectification column • Vacuum rectification column • Tray column • Packed column • Simple columns • Complicated columns Vacuum rectification • Under vacuum conditions • The less pressure the less boiling point of liquid • Vacuum distillation is used to distill or separate compounds that have a high boiling point or any compounds which might undergo decomposition on heating at atmospheric pressure.
• The vacuum is provided either by a water aspirator or by a mechanical pump. • Vacuum distillation is distillation at a reduced pressure.
• Since the boiling point of a compound is lower at a lower external pressure, • the compound will not have to be heated to as high a temperature in order for it to boil. • Vacuum gas oils – are used for producing of lubricating oils
• Vacuum residue - for producing of lubricating oils, building and construction bitumen, oil coke, road asphalts, etc.
• Adsorption – adsorbent, adsorptive • Absorption - absorbent, absorptive
• Desorption –
• Chemical sorption – is the process where sorption is occurred by chemical interaction between molecules • Gas absorption • (also known as scrubbing) is an operation in which a gas mixture is contacted with a liquid for the purpose of preferentially dissolving one or more components of the gas mixture and to provide a solution of them in the liquid.
|
|||||
|
Последнее изменение этой страницы: 2021-12-15; просмотров: 25; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.147.66.149 (0.01 с.) |