Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
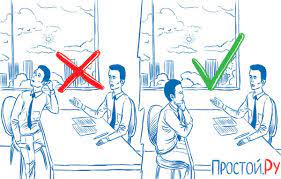
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Boiling Ranges - (temperature range) temperatures of the beginning and ending of fraction boiling point
• Fractions which are evaporated under 3000С • Mazut (bottom product) is residue after oil rectification and removing light distillations • Goudron is a vacuum residue after vacuum rectification of atmospheric residue • Ligth products, • Heavy products Lecture 2 Alkanes - C n H2 n +2 where n = 1,2,3,… • only single covalent bonds • saturated hydrocarbons (they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule) What is the structure of 2-propyl-4-methylhexane?
• Cycloalkanes (cycloparaffins) - CnH2n (n ≥ 3), • They include the basic mass of oil hydrocarbons • Saturated HC • Cyclic skeleton • Only single covalent bond. Lecture 3 • Naphtens have more higher melting points, boiling points and more bigger density then respective alkanes. Chemical reactions of naphthens • Less reactivity and very hard oxidable HC • 1. Hydrogenation reactions at high temperature • СН2 • Н2С СН2 + Н2 ® СН3-СН2-СН3 • 2. Dehydrogenation reactions for hexane HC Arenas • Unsaturated HC, ring type compounds with special cyclic group from six carbon atoms –benzene ring • All carbon atoms take part in stable single p - system formation by p-electrons • Arenas – are very desirable components of auto motor fuel, but not desirable for jet and diesel fuel in any kind • (Arenas concentrate in the medium fractions, its contain varies from 15 to 25%) • In gasoline fractions (under 200°С) are presented just benzene homologies. • In the crude oil are found all benzene and naphthalene homologies. • Phenyl - С6Н5 • С6Н5 -СН3 - methylbenzene (toluene) • phenylmethane • С6Н4 -(СН3) - ксилол • High thermal stability • Reactions of hydrogenation with naphtenes formation • Oxidize • 2С6Н6 + 15О2 →12СО2 +6Н2О. • • Crude oil also consist of various kind of HC which are formed by hybrid HC • They can contain aromatic cycles with alkyl substituted chains or aromatic HC with naphtenic cycles and others. • Indan, Tetralin, Fluoren - cycloalkanoarenas and their homologies are presence in diesel fractions • Sulfur containing compounds • Elemental sulfur S • Hydrogen Sulfide, H2S • Mercaptans R-SH • Sulfides (cyclic and non cyclic) R –S –R1 • Disulfides R –SS –R1 • More than 200 kinds of different sulfurous compounds • Mercaptanes (thiol) – R- S- H • R – radicals can be different (paraffinic, naphtanic, aromatic, hybrid est.) • Tengiz Oil – about 10% of mercaptanes • Mercaptanic sulfur are concentrated mostly in leading fractions • Light fractions b.b.p. - 600С consist of 85% of mercaptanes • Mercaptans are used in industry – odorant of gases (C2H5-SH-ethanethiol), in pharmacology, cosmetology • All types of SCC have neutral characteristics • • During oil refining SCC are hydrogenated with producing of hydrogen sulfide and then – go to getting the elemental sulfur and sulfuric acid • RSH + H2 = RH + H2S
|
||||
|
Последнее изменение этой страницы: 2021-12-15; просмотров: 26; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.139.83.7 (0.006 с.) |


 Reaction of condensation (with coke formation)
Reaction of condensation (with coke formation)