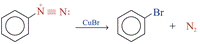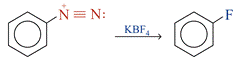Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Reductions of Amines with Nitric(III) Acid
• All amines react with nitric(III) acid • As nitric(III) acid is a weak and unstable acid, it is prepared by treating NaNO2 with an aqueous solution of a strong acid NaNO2(aq) + HCl(aq) ®¾ HNO2(aq) + NaCl(aq) 2NaNO2(aq) + H2SO4(aq) ®¾ 2HNO2(aq) + Na2SO4(aq)
Reductions of Primary Amines with Nitric(III) Acid Reaction of Primary Aliphatic Amines with Nitric(III) Acid • 1° aliphatic amines react with HNO2 to yield aliphatic diazonium salts • Diazonium salts, even at low temperatures, are highly unstable, they decompose spontaneously by losing nitrogen to form carbocations
Example:
Reaction of Primary Aromatic Amines with Nitric(III) Acid • 1° aromatic amines react with HNO2 to yield benzenediazonium salts • Benzenediazonium salts are unstable, but they do not decompose when the temperature of the reaction mixture is kept below 5°C
• The greater stability of benzenediazonium salts is due to the delocalization of the positive charge of the diazonium group + (–N º N) over the benzene ring
Reductions of Secondary Amines with Nitric(III) Acid • 2° aliphatic and aromatic amines react with HNO2 to yield N-nitrosoamines
Examples:
Reductions of Tertiary Amines with Nitric(III) Acid • 3° aliphatic amines don’t react with HNO2
Example:
6. Primary arylamines react with nitrous acid, HNO2, to yield stable arenediazonium salt • Arendiazonium salts are extremely useful in synthesis because the diasonio group (N2+) can be replaced by nucleophiles in asubstitution reaction
Preparation of Substituted Aromatic Compounds by Diazonio Replacement Reactions
Replacement of the Diazonium Group by –Cl, –Br or –CN (The Sandermeyer Reaction) The diazonium group of benzenediazonium ions can be replaced by –Cl, –Br and –CN by treating with CuCl, CuBr and CuCN respectively
Replacement of the Diazonium Group by –NO2 The diazonium group of benzenediazonium ions can be replaced by –NO2 by treating with NaNO2 in the presence of catalysts such as CuNO2
Replacement of the Diazonium Group by –SO2Cl The diazonium group of benzenediazonium ions can be replaced by –SO2Cl on reacting with SO2 in the presence of catalysts such as CuCl
Replacement of the Diazonium Group by –I The diazonium group of benzenediazonium ions can be replaced by –I on reacting with KI
Replacement of the Diazonium Group by –F The diazonium group of benzenediazonium ions can be replaced by –F on reacting with KBF4
|
||||
|
Последнее изменение этой страницы: 2017-02-07; просмотров: 274; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.224.246.203 (0.005 с.) |








 Arendiazonium Salts
Arendiazonium Salts

 ,
,