Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Methods of genetic engineering: agrobacterial genetic transformation ⇐ ПредыдущаяСтр 4 из 4
The ability of Agrobacterium to transfer genes to plants and fungi is used in biotechnology, in particular, genetic engineering for plant improvement. A modified Ti or Ri plasmid can be used. The plasmid is 'disarmed' by deletion of the tumor inducing genes; the only essential parts of the T-DNA are its two small (25 base pair) border repeats, at least one of which is needed for plant transformation. Marc Van Montagu and Jozef Schell at the University of Ghent (Belgium) discovered the gene transfer mechanism between Agrobacterium and plants, which resulted in the development of methods to alter Agrobacterium into an efficient delivery system for gene engineering in plants.[7][8] A team of researchers led by Dr Mary-Dell Chilton were the first to demonstrate that the virulence genes could be removed without adversely affecting the ability of Agrobacterium to insert its own DNA into the plant genome (1983). The genes to be introduced into the plant are cloned into a plant transformation vector that contains the T-DNA region of the disarmed plasmid, together with a selectable marker (such as antibiotic resistance) to enable selection for plants that have been successfully transformed. Plants are grown on media containing antibiotic following transformation, and those that do not have the T-DNA integrated into their genome will die. An alternative method is agroinfiltration. Transformation with Agrobacterium can be achieved in two ways. Protoplasts, or leaf-discs can be incubated with the Agrobacterium and whole plants regenerated using plant tissue culture. A common transformation protocol for Arabidopsis is the floral-dip method: the flowers are dipped in an Agrobacterium culture, and the bacterium transforms the germline cells that make the female gametes. The seeds can then be screened for antibiotic resistance (or another marker of interest), and plants that have not integrated the plasmid DNA will die. Agrobacterium does not infect all plant species, but there are several other effective techniques for plant transformation including the gene gun. 63) Collection and cultivation of oocytes in vivo and in vitro One way to obtain a large number of descendants of genetically valuable livestock females - oocytes fertilization and embryo culture outside the body (in vitro). Traditional methods (in vivo) reproduction of farm animals females produce significantly fewer gametes than males. The problem of oocyte maturation and fertilization in vitro, followed by culturing embryos and their non-surgical transplant recipients and receiving numerous progeny definitively solved. Fertilization in vivo matured oocytes in the ovary, when considered in isolation from the maturation of oocytes in vitro, is of limited use for the breeding of farm animals. In this case, as a result of induction of superovulation surgically or during slaughter cows can be obtained in vivo matured units full oocytes. Only a combination of fertilization of oocytes matured in vitro followed by transplantation of embryos can significantly increase the use of the genetic potential of female gametes. So, if after induction poliovulyatsii per cow - get up to 10 donor tubalnyh, ie matured in vivo, and ovulated oocytes from the ovary is cow can be removed to 200 follicular oocytes. Oocyte maturation by culturing reached 80% or higher, and their fertility - 50-70%. It is established that in vitro maturation of oocytes not equivalent natural that occurs in vivo in the oocyte prior to ovulation. As shown by numerous studies, the in vitro maturation of the kernel is without the full cytoplasmic maturation. Under the cytoplasmic maturation understand set of morphological, biochemical and physiological changes in the oocyte cytoplasm, resulting in the egg becomes fertilized and capable of further development. By culturing the oocytes in vitro maturation of immature to understand the process of oocytes in artificial media in which immature oocytes undergo meiotic maturation to metaphase of the second division, that is, to the stage of readiness for fertilization.
To isolate the oocytes from follicles, as a rule, use the ovaries of slaughtered cows and less ovaries extracted operational putem.Posle oocyte retrieval of antral follicle premises in the culture medium is a first morphological changes associated with the nuclear maturation of oocytes, ie meiosis resumes. The first morphological sign of maturity - the destruction of the germinal vesicle - oocyte nuclei, ie prophase oocyte nucleus is converted to a meta-phase. The first meiotic division, which is a pressure reducer, is replaced by the second equational division in which there is a division of chromosomes in mitosis and spindle formation of ahromatino of new yarns. At this stage of meiotic maturation division is blocked until the oocyte fertilization. On the completion of oocyte maturation in vivo or in vitro judged by the appearance of the polar body or towards the oocyte-mi metaphase of the second division sozrevaniya.Intensivny synthesis, carried out by the oocyte is associated with gene activity and an increase in the number of copies. Found that the first stage of embryonic development take place in an environment where the core does not produce RNA in the cytoplasm, and the process is controlled by RNA accumulated in oogenesis. The obtained results show that changes in protein synthesis are not associated with nuclear and cytoplasmic maturation of crucial importance for normal fertilization and early embryo development up to the implantation of the embryo in the uterine wall of the recipient. In connection with this, methods for enhancing the synthesis of RNA and protein during maturation of oocytes in vitro. It is assumed that during the maturation of oocytes in vitro in the cytoplasm are not fully synthesized factor responsible for the formation of the male pronucleus.
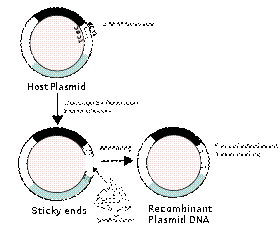
Draw a diagram of an experiment in genetic engineering (design recDNA) and give a description of the main stages Molecular cloning is the laboratory process used to create recombinant DNA. It is one of two widely used methods (along withpolymerase chain reaction, abbr. PCR) used to direct the replication of any specific DNA sequence chosen by the experimentalist. The fundamental difference between the two methods is that molecular cloning involves replication of the DNA within a living cell, while PCR replicates DNA in the test tube, free of living cells. Formation of recombinant DNA requires a cloning vector, a DNA molecule that will replicate within a living cell. Vectors are generally derived from plasmids or viruses, and represent relatively small segments of DNA that contain necessary genetic signals for replication, as well as additional elements for convenience in inserting foreign DNA, identifying cells that contain recombinant DNA, and, where appropriate, expressing the foreign DNA. The choice of vector for molecular cloning depends on the choice of host organism, the size of the DNA to be cloned, and whether and how the foreign DNA is to be expressed.[5] The DNA segments can be combined by using a variety of methods, such as restriction enzyme/ligase cloning or Gibson assembly. In standard cloning protocols, the cloning of any DNA fragment essentially involves seven steps: (1) Choice of host organism and cloning vector, (2) Preparation of vector DNA, (3) Preparation of DNA to be cloned, (4) Creation of recombinant DNA, (5) Introduction of recombinant DNA into the host organism, (6) Selection of organisms containing recombinant DNA, (7) Screening for clones with desired DNA inserts and biological properties.
Construction of recombinant DNA, in which a foreign DNA fragment is inserted into a plasmid vector. In this example, the gene indicated by the white color is inactivated upon insertion of the foreign DNA fragment.
|
|||||
|
Последнее изменение этой страницы: 2016-08-06; просмотров: 200; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.191.176.66 (0.009 с.) |