Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
TASK 6. Give your own opinion on the text
______________________________________________________________________________________________________________________________________________________________________________________________________ ______________________________________________________________________________________________________________________________________________________________________________________________________ TEXT 5 THE NATURE OF THE ATOM There is one source of energy, however, which owes nothing to Today we know that atoms are neither unchangeable nor indivisable. It may be sufficient to recall that Marie and Pierre Curie, by their discovery of radium, in 1898, made the whole theory of the indivisible atom crumble, Another discovery, made three years earlier, seemed to point in the same direction: that of the X-rays by Professor Wilhelm Konrad Rontgen at the University of Bavaria. These and other phenomena and discoveries around the turn of the century Hydrogen, for instance, being the lightest; and simplest element, has only one of each; uranium, the heaviest element occurring in Nature, has 92. Theoretically, we could change lead into gold, as the alchemists dreamed of doing, by removing three protons and electrons from a few billion lead atoms, which have 82 of each, then we would get gold atoms with 79 protons and electron each. However, the knocking-off process would be much more expensive than the gold we would get. The neutrons, which are present in the atoms of many elements, are of particular importance in the utilization of atomic energy. Most elements are mixtures of ordinary atoms and so-called isotopes: the isotope atoms have more, or fewer, neutrons than the ordinary atoms. An isotope differs from the ordinary form of the element only in weight, but chemically it behaves in exactly the same way. Water, for instance, is a mixture of ordinary molecules of hydrogen and oxygen atoms and of 'heavy' ones. The heavy hydrogen atom has an extra neutron in its nucleus. Uranium, on the other hand, Curies discovered in radium an unstable nucleus is one that is likely to break up into the nucleus of another element. Professor Otto Hahn found in Berlin in 1938 that when uranium atoms are bombarded with neutrons they split up in a process which he called 'fission' (a term used in biology for the way in which some cells divide to form new ones). The 92 protons of the uranium nucleus split up into barium, which has 56, and krypton, a gas with 26 protons. Frederic Joliot-Curie, the son-in-law of Marie Curie, proved some months later that in this fission process some neutrons from the uranium nucleus were liberated; they flew off, and some struck other nuclei, which in turn broke up, liberating still more neutrons. Enrico Fermi, an Italian who liad gone to America to escape life under fascism, developed the theory of what would happen if a sufficiently large piece of unstable uranium broke up in this way — there would be a 'chain reaction': the free neutrons would be bombarding the nuclei with such intensity that in no time at all
This was the theory that led, within the short space of four years, to the first atom bombs. On Monday, 6 August 1945, while cheerful crowds in England enjoyed their first holiday after the end of the war in Europe, one such bomb was dropped on the town of Hiroshima in Japan. It killed or injured nearly 200,000 people. Three days later another bomb was dropped on Nagasaki, with 65,000 victims. The centres of both cities were completely destroyed. PHRASES & WORD COMBINATIONS TO THE TEXT 1. which owes nothing to – который не зависит от… 2. …made the whole theory of the indivisible atom crumble – разрушили теорию неделимости атома 3. around the turn of the century – на грани двух веков 4. which have next to no mass and weight – которые почти не имеют ни массы, ни веса 5. on the other hand – с другой стороны 6. in no time at all – мгновенно EXERCISES TASK 1. Answer the questions: 1. What is one of the numerous sources of energy that owes nothing to the heat and light of the sun? _____________________________________________________ __________________________________________________________________ 2. What is the story of research into the nature of atom? _____________________ __________________________________________________________________ 3. Who found that the radiation was able to penetrate thin matter like wood and human flesh? _______________________________________________________ __________________________________________________________________ 4.What have you known about Special Theory of Relativity? _________________ __________________________________________________________________ 5.What is the atom? Is it a miniature solar system? _________________________ __________________________________________________________________ 6.What does the atom consist of? _______________________________________ __________________________________________________________________ 7. What is an ion? ___________________________________________________ 8. Do the atoms of all the elements contain the same kind of particles? __________ __________________________________________________________________ 9. What is of particular importance in the utilization of atomic energy? _________ __________________________________________________________________ 10.What did Curies discover in radium? __________________________________ __________________________________________________________________ TASK 2. Match the equivalents: Protons, electrons, solar system, Theory of Relativity, phenomena, particles, discovery, unchangeable, atomic nucleus, source of energy. ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
электроны неизменный теория относительности атомное ядро феномен источник энергии солнечная система протоны открытие частицы TASK 3. Fill in the blanks with words and word combinations from the text: The atoms of all the _______ contain the _______ of particles; what distinguishes them from each other is merely the ________ — of protons in the _______ and of _______ revolving around it. Hydrogen, for instance, being the lightest; and _________, has only one of each; uranium, the _______- occurring in Nature, has ________. So all you have to do to change one ________ into another is either to knock some _______ and a corresponding number of ________ off each atom, or add them; in fact, this _______ is going on in Nature all the time. TASK 4. Continue the sentenses according to the text: 1. Today we know that atoms are_______________________________________ __________________________________________________________________ 2. Marie and Pierre Curie, by their discovery of radium, in 1898, made the whole theory___________________________________________________________ __________________________________________________________________ 3. Using a cathode-ray tube, he found____________________________________ __________________________________________________________________ 4. These and other phenomena and discoveries around______________________ __________________________________________________________________ 5. Danish assistant, Niels Bohr, developed________________________________ __________________________________________________________________ 6. The atoms of all the elements contain__________________________________ __________________________________________________________________ 7. Most elements are_________________________________________________ __________________________________________________________________ 8. Curies discovered in radium_________________________________________ __________________________________________________________________ 9. Frederic Joliot-Curie_______________________________________________ __________________________________________________________________ 10. This was the theory that led_________________________________________ __________________________________________________________________ TASK 5. Translate into English: Атомы всех элементов содержат одни и те же частицы; что отличает их друг от друга - количество частиц - протонов в ядре и электронов, вращающихся вокруг него. Теоретически, мы могли бы превратить свинец в золото, как мечтали алхимики с помощью удаления трех протонов и электронов из нескольких миллиардов атомов свинца, мы получим атомы золота с 79 протонами и электронами в каждом. ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
|
||||||
|
Последнее изменение этой страницы: 2021-02-07; просмотров: 115; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.118.227.69 (0.013 с.) |
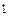 the heat and light of the sun; nor can it be harnessed by a chemical process. It is the energy of the atomic nucleus.
the heat and light of the sun; nor can it be harnessed by a chemical process. It is the energy of the atomic nucleus.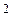 because here was an element which disintegrated and sent out rays, consisting of particles much smaller than the atom.
because here was an element which disintegrated and sent out rays, consisting of particles much smaller than the atom.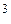 were deeply disturbing for the physicists, and they saw that the whole traditional concept of the structure of matter had to be completely revised. Wnen, as early as 1905, Albert Einstein published his Special Theory of Relativity, in which he declared that matter could be converted into energy — very little matter into very great energy — there was a storm of protest in the scientific world. But little by little the evidence that he was right accumulated, and within a few years an entirely new picture of the atom emerged from the studies and laboratories of scientists in many countries. From that evidence Lord Rutherford, the New Zealand-born scientist, and his young Danish assistant, Niels Bohr, developed by 1911 their revolutionary theory of what the atom was really like. The electrons, which have next to no mass and weight,
were deeply disturbing for the physicists, and they saw that the whole traditional concept of the structure of matter had to be completely revised. Wnen, as early as 1905, Albert Einstein published his Special Theory of Relativity, in which he declared that matter could be converted into energy — very little matter into very great energy — there was a storm of protest in the scientific world. But little by little the evidence that he was right accumulated, and within a few years an entirely new picture of the atom emerged from the studies and laboratories of scientists in many countries. From that evidence Lord Rutherford, the New Zealand-born scientist, and his young Danish assistant, Niels Bohr, developed by 1911 their revolutionary theory of what the atom was really like. The electrons, which have next to no mass and weight,  are negatively charged; in fact, they are the carriers of electricity in all our electric wires and appliances. Normally there are as many positive protons in the nucleus as there are electrons revolving around it, so that their charges cancel each other out and the atom as a whole is electrically neutral.
are negatively charged; in fact, they are the carriers of electricity in all our electric wires and appliances. Normally there are as many positive protons in the nucleus as there are electrons revolving around it, so that their charges cancel each other out and the atom as a whole is electrically neutral. has an isotope whose nucleus contains fewer neutrons than the ordinary element. This isotope — atomic weight: 235; atomic weight of ordinary uranium: 238 – has a very special significance in nuclear physics because it is, like many other heavy-element isotopes, 'unstable'.
has an isotope whose nucleus contains fewer neutrons than the ordinary element. This isotope — atomic weight: 235; atomic weight of ordinary uranium: 238 – has a very special significance in nuclear physics because it is, like many other heavy-element isotopes, 'unstable'. the whole lump of uranium would disintegrate.
the whole lump of uranium would disintegrate.