Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Formulas for imaging of stereo isomers
Conformational Isomerism Conformational isomers are stereoisomers that have different arrangements of their atoms in space due to free rotation about a covalent σ-bond. e.g.
Geometrical Isomerism Geometrical isomers are stereoisomers that have different arrangements of their atoms in space due to restricted rotation about a covalent bond.
Geometrical isomers have different physical and chemical properties.
trans -But-2-ene has higher melting point Þ more regular and symmetrical structure Þ molecules pack more compactly in crystal lattice Þ difficult to break the lattice Þ higher melting point
cis -But-2-ene has higher boiling point because it has net dipole moment
Þ molecules are held together by dipole-dipole interactions Þ trans -isomer has no net dipole moment, their molecules are held by instantaneous dipole-induced dipole interactions Þ dipole-dipole interactions are stronger Þ cis -but-2-ene has higher boiling point Another example: cis -butenedioic acid and trans -butenedioic acid
trans -butenedioic acid has higher melting point While the cis -isomer forms intramolecular hydrogen bonding
Although trans -butenedioic acid can form more extensive hydrogen bonds with water molecules, cis- butenedioic acid is more soluble in water than the trans -isomer because of the greater dipole moment.
cis - and trans -butenedioic acids have different chemical properties
Enantiomerism • Enantiomerism occurs in those compounds whose molecules are chiral. • A chiral molecule is one that is not superimposable with its mirror image. • The chiral molecule and its mirror image are enantiomers.
sp 3-hybridized carbon atom with two or more identical groups attached is achiral and contains a plane of symmetry sp 3- hybridized carbon atom with four different groups attached is chiral and do not contain a plane of symmetry E.g. butan-2-ol
|
||||||||||||||||||||||
|
Последнее изменение этой страницы: 2017-02-07; просмотров: 214; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.149.26.246 (0.006 с.) |


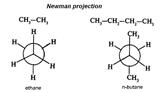

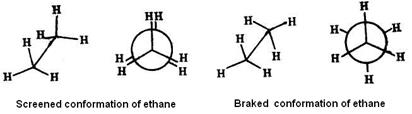

 Conformational isomers interconvert into each other without breaking of chemical bonds. Unlike structural isomers, different conformers can’t usually be isolated, because theyinterconverttoo rapidly. This kind of isomers can be fined by physical and chemical methods only.
Conformational isomers interconvert into each other without breaking of chemical bonds. Unlike structural isomers, different conformers can’t usually be isolated, because theyinterconverttoo rapidly. This kind of isomers can be fined by physical and chemical methods only. Rotation of a carbon atom of a C = C bond through an angle of 90° causes the breaking of the p bond.
Rotation of a carbon atom of a C = C bond through an angle of 90° causes the breaking of the p bond.