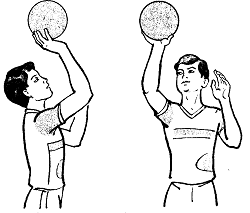
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
The basic photographic process
A simple black and white film consists of the film base and the emulsion layer. The emulsion is the light-sensitive layer of the film. It is made up of small crystals of sensitive material evenly distributed in a support medium. The light-sensitive medium is silver halide, derived from pure silver, and the support medium is gelatine, which is made from animal skins and bones. The gelatine support medium has a number of very useful proper- ties. It is more or less transparent and therefore will let light through to all the sensitive crystals at whatever depth they are embedded in its thickness. It is easily permeated by water, so the various chemicals that are needed to act upon the crystals during processing can reach their target. It is also reasonably easy to produce in a very pure form.
You have heard of a photographic emulsion as being made up of grains and, indeed, we often refer to the visual texture of a photographic image as being either grainy or, perhaps, having fine grain. The chemical construction of a photographic emulsion starts with a single atom of silver halide; these atoms are then grouped together in varying numbers, some forming small clusters made up of few atoms and some forming large clusters containing many atoms. A typical emulsion will have many sizes of clusters, from really quite large to very small. All of these clusters, whatever their size, are known as the grains in the film. If part of the photographic image is made up of many small grains, then it might be referred to as being fine grain. This means that there are so many small grains packed so close together that in the final image the human eye will find it very hard to discern one grain from another and the picture, over that area, will look as if it has a continuous smooth tone. If another part of the image is made up of a few large grains, each probably spaced some distance apart, then the eye might be just able to discern the pattern of the grains, and then it will not look very like a continuous tone and we would therefore describe it as grainy. But how does all this come about?
If you look at a picture of the process, you will you will see a representation of the layout of different sized grains embedded in the emulsion before any exposure has taken place. To make the following explanation easier, let us suppose that we have only four different sizes of grain shown in the illustration; in reality, there would be a huge number of different sizes of grains. For the propose of understanding more clearly, let us consider one grain of each of the four sizes in our example being hit by an even area of light. Light is made up of particles of energy we refer to as photons. These photons attack the grains. Because the photons are evenly spaced and the grains are of differing sizes, the number of photons to hit each grain will differ. What will happen next is a chemical reaction?
We have seen that below the grain size there is a molecular structure. Let us suppose, for the sake of argument, that every photon strikes an atom of the silver halide. If this is the case, then every photon will cause an atom of silver halide to convert to an atom of metallic silver. This is the first part of the photographic process. The emulsion will now consist of a number of silver halide atoms and a number of metallic silver atoms. Clearly, we could not see this image as our eyes would need light for that to happen and that would immediately expose every atom in the emulsion. This is, in effect, an image waiting to be made visible and we therefore refer to it as a latent image. In order to make the image usable, again in total darkness, we immerse the film in a liquid known as the developer. What happens next is only slightly short of miraculous.
The developer is very selective, for if it is applied for the right amount of time, in the correct strength and at the right temperature, it will search out grains containing four or more atoms of metallic silver within them and turn all the atoms to metallic silver, not just those atoms of metallic silver but every atom of silver halide within that grain as well. Grains, however big or small, that have three or less grains of metallic silver will be totally unaffected, not even those atoms that have been turned to metallic silver will become black. Still in total darkness, and after exactly the right amount of development time, we plunge our film into an acid stop bath. As its name suggests, this immediately stops the process of development but still leaves the image vulnerable to more light.
After the acid stop bath, there remain active silver halide grains in the film so, still in complete darkness, the film must now go into a fix bath, which turns the remaining silver halide into substance (complex sodium argentothiosulphates) that can then be washed out of the emulsion without affecting the metallic silver. This chemical, the fixer, is the sodium hyposulphate discovered by Sir John Herschel in 1839, referred to earlier in this chapter. You will occasionally hear the fixer referred to as “hypo”, a name derived from the chemical name of its primary component. So, if the fixer dissolves out of the photographic emulsion all the undeveloped grains but leaves all the developed grains completely unaffected, then our little microcosm of the film will, at last, be safe to bring into the light. The application of all the above liquid chemicals to the emulsion is referred to as processing that emulsion.
If the film is to remain in perfect condition for many years it must now be washed for a considerable time, as all the unwanted chemicals must be completely removed and the gelatine emulsion left with only metallic silver to form the image. Therefore, the image formed by this process will have dark areas where much light fell upon the film and light areas where little light arrived and, fortunately for our purpose, all the shades of grey in between. It will therefore be the reverse brightness of the original scene, and this we call a negative image. In order to obtain an image that represents the original scene, the negative must be printed on to another piece of film, just as Fox Talbot’s process announced in 1839. This new film will produce an image with the reverse densities of the negative and will return to the values corresponding to the original scene. This new film is called the positive and must be processed in exactly the same way as the camera negative.
Colour negative film
There is no such thing as a single colour photographic emulsion. At its simplest, a modern colour negative film is made up from three black and white emulsions so arranged that each single layer records the image of one of the primary colours that make up white light, i.e. red, green and blue. The layers of coatings on the film base are arranged in order to record a colour image in what is known as an integral tripack colour emulsion. Under the supercoat is the first emulsion. It is relatively easy to make a black and white emulsion that is only sensitive to blue light, so this is used as the uppermost emulsion layer. Underneath this coating is an optical filter layer coloured yellow. A yellow filter will let green and red light through, but not blue. Again, it is possible to make a black and white emulsion that is only sensitive to green and blue light, but all the blue light has now been filtered out, so this layer will only be struck by the green and red light passed by the yellow filter. It will, therefore, only record the green part of the image. The bottom emulsion layer is sensitive to red and blue, but not to green, so this layer can only respond to red as blue has been filtered out. If an exposed film of this type is simply immersed in the standard black and white developer/fixer process, all you will get is three black and white images.
Therefore, a clever bit of chemistry is incorporated in each of the three emulsions. The chemicals used are known as colour couplers. Each individual layer has embedded in it a different colour coupler whose job is, at a molecular level, to form a dye of the colour complementary to the colour of the light that exposed the layer. What is more, this dye is formed only where silver is formed by the developer. As this is a negative film intended for printing, each layer of emulsion must end up dyed the complementary colour to the original scene. Therefore: The blue-sensitive layer forms yellow dye, the green-sensitive layer forms magenta dye, the red-sensitive layer forms cyan dye. Early colour film was capable of recording roughly seven stops of tonal range or a brightness range of 128:1. Modern film stocks have now been introduced with a tonal range of around 10 stops or a brightness range of over 1000:1.
Grain and graininess Grain is the texture we see in a print that appears as a texture not associated with the original scene. It becomes apparent when, for various reasons, we can begin to perceive the distribution of the developed metallic silver particles and we can perceive them long before we can actually see any particles. Furthermore, because the print stock is of exceptionally fine grain, and consequently has a very slow speed, the perceived grain comes from the structure of the negative emulsion, not the positive emulsion.
It is thought that a larger crystal of silver halide is more sensitive to light mainly because its surface area is greater and therefore has a bet- ter chance of catching a light ray and being exposed. It follows that a film emulsion with a preponderance of large grains will therefore be more sensitive. A fast, highly sensitive film emulsion will normally appear grainy. Conversely, a fine-grained film emulsion has a high resolution and poor sensitivity. At this point it must be said that recent developments in emulsion technology have made the perceivable differences between film stocks of differing speeds much less noticeable than they were even a few years ago.
It is worth considering at this point the difference between resolution and perceived sharpness as it relates to the film emulsion. Resolution is defined as the ability of the emulsion to record very narrow bands of black and white lines and is measured, therefore, as the maximum number of lines per millimetre that can be recorded on the emulsion’s surface. Acutance, or what actually looks sharp to the eye, is the ability to record the edge sharpness of an image. Difference in grain size and the contrast of the image will change the relationship of the apparent definition and acutance of a given film stock, and must always be carefully balanced by the emulsion designer. Resolution, acutance and apparent sharpness do not, therefore, always go hand in hand.
Camera filters
We use filters in cinematography to alter the image either for technical reasons to correct the colour of the light to that required by the film we are using, or because we may wish to change the image in some way that will enhance our storytelling powers. The former is often neces- sary, the latter much more fun.
The majority of coloured filters are known by numbers or a combination of numbers and letters – for instance, 85 and 85B.These are sim- ply the catalogue number they are to be found under in the Eastman Kodak Wratten Filter Catalogue. All filter manufacturers will use the Wratten filter numbers for their version of the filter in their list that conforms to the transmission characteristics of the filter in the Wratten list. A Wratten catalogue complete with transmission graphs can be an illuminating read once you know how to interpret the information. Effects filters are usually described by some term that indicates what change they will make to the image – for example, fog filter.
Colour-compensating filters
Colour-compensating filters, or CC filters, are carefully stepped filters in the primary colours or their reciprocal colour. They are used, in front of the lens, to correct the source light to the film in use. They come in small steps so you need a lot of them to be able to cope with any situation. In reality, we rarely use CC filters in cinematography – they are mainly used in still photography to obtain an accurate original trans- parency, as with the reversal process there can be no correction later.
Colour-correction filters
This phrase usually refers to filters used to correct a daylight scene when shooting with tungsten-balanced film. The usual full correction filter is the Wratten 85.The various alternatives are: Wratten 85 Full correction 85B Full correction slight warming – often used as standard correction when filming for telecine, as this can enhance the transfer to tape 85C Half the correction of the straight 85 – often used when interior is lit to the same colour balance, so that any exterior, say through a window, appears slightly cold.
All these filters also come with a neutral density built into them. For the 85 these are available as 85BN3, 85BN6 and 85BN9. Each unit of 3 stands for a density of 0.3, which exactly halves the light passing through the filter. Therefore, an 85BN3 reduces the exposure by an extra stop in addition to the two-thirds of a stop correction needed for the straight 85. Likewise, the 85BN6 reduces the exposure by two stops and the 85BN9 by three stops, again in addition to the straight 85.
Skin tone warmer A Tiffen 812 is very effective in warming up skin tone while having a negligible effect on the other colours in the scene. This is particularly useful for a close-up on a cold day.
|
||||||
|
Последнее изменение этой страницы: 2017-01-20; просмотров: 292; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.219.22.169 (0.012 с.) |