Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
General Organic Chemistry (2)Стр 1 из 4Следующая ⇒
Lecture 2 General Organic Chemistry (2)
• Structural Isomerism • Stereoisomerism Types of Organic Reactions There are four possible of alkyl substitution for carbon denoted 10 (primary), 20 (secondary), 30 (tertiary) and 40 (quaternary) e.g,
1.1Classification of organic reactions by type of chemical bonds braking Homolysis
The movement of a single electron to each atoms with formation two free radicals. Energy must be supplied in, either in form of heat or irradiation with light. e.g. chlorine undergoes homolysis readily when heated, or when irradiated with light of a wavelength that can be absorbed by the molecule to form two chlorine radicals
General equation of homolysis of a bond to carbon:
e.g. methane undergoes homolysis to form a methyl radical and a hydrogen radical
Free Radicals • Electrically neutral atoms or groups of atoms possessing an unpaired electron • Highly reactive because of the unstable electronic configuration e.g.
tert -butyl carboradical is the most stable because electron-donating groups exert positive inductive effects and hyperconjugation effects to stabilize carbon atom. The greater number of alkyl groups attached to the central carbon atom, the more stable is the carboradical.
If the carbon atom with the unpaired electron is related to the atom in a state of sp2-hybridization or with a heteroatom which has a lone pair of electrons, carboradical is more stable due to the delocalization of charge on the conjugating system (р-π-conjugation or р-р-conjugation)
Heterolysis
The movement of a pair of electrons to one atom. Two charged fragments or ions formed. Heterolysis of a bond requires the bond to be polarized.
The greater the difference in electronegativity between the atoms, the greater is the polarization of the bonds. The product of heterolysis of a bond to carbon depends on the electronegativity of the atom that is bonded to the carbon atom.
tert -butyl carbocation is the most stable because electron-donating groups exert positive inductive effects to reduce the positive charge on the carbon atom. Like carboradical, the greater number of alkyl groups attached to the central carbon atom, the more stable is the carbocation.
tert -butyl carboanion is the least stable because electron-donating groups exert positive inductive effects to increase the negative charge on the carbon atom. Unlike carbocation and carboradical, the greater the number of alkyl groups attached to the central carbon atom, the less stable is the carboanion.
Electrophiles • electron-deficient species that tend to accept electron(s) • possess an empty orbital to receive the electron pair • cations or free radicals seeking electron-rich centres Nucleophiles • electron-rich species that tend to seek an electron-deficient site for reaction • possess lone pairs of electrons • anions or molecules with lone pairs of electrons
Substitution Reactions
An atom or a group of atoms of the reactant molecule is replaced by another atom or group of atoms. Characteristic reactions of saturated compounds e.g.
Addition Reactions Two molecules react to give a single product. Characteristic reactions of compounds with multiple bonds. e.g.
Elimination Reactions Atoms or groups of atoms are removed from two adjacent atoms of the reactant molecule. Method for preparing compounds with multiple bonds. e.g.
Condensation Reactions Two or more molecules join together, with a small molecule being removed e.g.
Rearrangement Reactions A molecule undergoes reorganization of its constituent atoms or groups of atoms e.g.
Isomerism
Structural Isomerism
Chain Isomerism Chain isomers are isomers that have different carbon skeletons.
Position Isomerism Position isomers are isomers that have the same carbon skeleton and functional group. They differ only in the position of the functional group.
Metamerism Metamers are those isomers with the functional group interrupting the carbon skeleton at different positions.
Tautomerism Tautomers are those isomers with structures differing in arrangement of atoms. They are in dynamic equilibrium with each other.
Functional Group Isomerism Functional group isomers are isomers that have the same molecular formula but contain different functional groups.
Stereoisomerism Conformational Isomerism Conformational isomers are stereoisomers that have different arrangements of their atoms in space due to free rotation about a covalent σ-bond. e.g.
Geometrical Isomerism Geometrical isomers are stereoisomers that have different arrangements of their atoms in space due to restricted rotation about a covalent bond.
Geometrical isomers have different physical and chemical properties.
trans -But-2-ene has higher melting point Þ more regular and symmetrical structure Þ molecules pack more compactly in crystal lattice Þ difficult to break the lattice Þ higher melting point
cis -But-2-ene has higher boiling point because it has net dipole moment
Þ molecules are held together by dipole-dipole interactions Þ trans -isomer has no net dipole moment, their molecules are held by instantaneous dipole-induced dipole interactions Þ dipole-dipole interactions are stronger Þ cis -but-2-ene has higher boiling point Another example: cis -butenedioic acid and trans -butenedioic acid
trans -butenedioic acid has higher melting point
While the cis -isomer forms intramolecular hydrogen bonding
Although trans -butenedioic acid can form more extensive hydrogen bonds with water molecules, cis- butenedioic acid is more soluble in water than the trans -isomer because of the greater dipole moment.
cis - and trans -butenedioic acids have different chemical properties
Enantiomerism • Enantiomerism occurs in those compounds whose molecules are chiral. • A chiral molecule is one that is not superimposable with its mirror image. • The chiral molecule and its mirror image are enantiomers.
sp 3-hybridized carbon atom with two or more identical groups attached is achiral and contains a plane of symmetry sp 3- hybridized carbon atom with four different groups attached is chiral and do not contain a plane of symmetry E.g. butan-2-ol
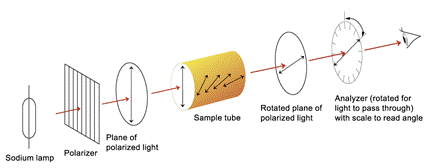
Plane-polarized Light Light is an electromagnetic radiation.
Polarimeter is a device used for measuring the effect of optically active compounds on plane-polarized light.
If the tube of polarimeter is empty, or if an optically inactive substance is present, the axes of the plane-polarized light and the analyzer will be exactly parallel. If the tube contains an optically active substance, the plane-polarized light will rotate as it passes through the tube. If the analyzer is rotated in a clockwise direction, the rotation is said to be positive (+). If the rotation is anticlockwise, the rotation is said to be negative (–). Molecule whith one chiral carbon atom exists informs of two stereoisomers. A compound with n chiral atoms can have a maximum of 2 n stereoisomers. N = 2n, where N – number of optical isomers; n – number of chiral atoms e.g. N = 22 = 4
Molecule having two chiral centers with identical substituent exists in three stereoisomeric forms. Because one isomer has a symmetry plane and is achiral. Compounds that are achiral, yet contain stereogenic centers, are called meso compounds (meso forms).
Tartaric acid exists in three stereoisomeric forms: two enantiomers and one meso form.
Racemic mixtures, or racemates, are 50:50 mixtures of (+) and (-) enantiomers. Racemic mixtures and individual diastereomers differ in their phisical properties, such as solubility, melting point, and boiling point. Most reactions give chiral prodacts. If the reactant are optically inactive, the products are also optically inactive – either meso or racemic. If one ore both of the reactant is optically active, the product can also be optically active. Questions and problems 1. Give the symbol for the first-order nucleophile addition reaction а) ЕS1; b) NA1; c) AN1; d) AE1; e) SN2; f) E; g) SE1. 2. What kind of reaction is the following one? 3. Give the symbol for the following reaction 4. Dispose the carbocations as their stability grows: 1) 5. What kind of isomerism is there in the following pair of compounds 6. What kind of formulas was used in the previous question? а) full structural; b) short structural; c) simplified structural; d) sterechemical; e) perspective; f) Newman formula; g) Fischer formula. 7. What kind of isomers has the same empirical formulas but different functional groups? а) chain isomerism; b) position isomerism; c) metamerism; d) tautomerism; e) conformational isomerism; f) enantiomerism; g) geometrical isomerism. 8. Lecture 2 General Organic Chemistry (2)
• Structural Isomerism • Stereoisomerism Types of Organic Reactions There are four possible of alkyl substitution for carbon denoted 10 (primary), 20 (secondary), 30 (tertiary) and 40 (quaternary) e.g,
1.1Classification of organic reactions by type of chemical bonds braking Homolysis
The movement of a single electron to each atoms with formation two free radicals. Energy must be supplied in, either in form of heat or irradiation with light. e.g. chlorine undergoes homolysis readily when heated, or when irradiated with light of a wavelength that can be absorbed by the molecule to form two chlorine radicals
General equation of homolysis of a bond to carbon:
e.g. methane undergoes homolysis to form a methyl radical and a hydrogen radical
Free Radicals • Electrically neutral atoms or groups of atoms possessing an unpaired electron • Highly reactive because of the unstable electronic configuration e.g.
tert -butyl carboradical is the most stable because electron-donating groups exert positive inductive effects and hyperconjugation effects to stabilize carbon atom. The greater number of alkyl groups attached to the central carbon atom, the more stable is the carboradical.
If the carbon atom with the unpaired electron is related to the atom in a state of sp2-hybridization or with a heteroatom which has a lone pair of electrons, carboradical is more stable due to the delocalization of charge on the conjugating system (р-π-conjugation or р-р-conjugation)
Heterolysis
The movement of a pair of electrons to one atom. Two charged fragments or ions formed. Heterolysis of a bond requires the bond to be polarized.
The greater the difference in electronegativity between the atoms, the greater is the polarization of the bonds. The product of heterolysis of a bond to carbon depends on the electronegativity of the atom that is bonded to the carbon atom.
tert -butyl carbocation is the most stable because electron-donating groups exert positive inductive effects to reduce the positive charge on the carbon atom. Like carboradical, the greater number of alkyl groups attached to the central carbon atom, the more stable is the carbocation.
tert -butyl carboanion is the least stable because electron-donating groups exert positive inductive effects to increase the negative charge on the carbon atom. Unlike carbocation and carboradical, the greater the number of alkyl groups attached to the central carbon atom, the less stable is the carboanion.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Последнее изменение этой страницы: 2017-02-07; просмотров: 245; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.149.230.44 (0.12 с.) |








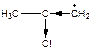 Electron-accepting groups exert negative inductive effects to destabilize carboradical due to increase of electron deficit on the carbon atom.
Electron-accepting groups exert negative inductive effects to destabilize carboradical due to increase of electron deficit on the carbon atom.






 Electron-accepting groups exert negative inductive effects to destabilize carbocation due to increase of electron deficit on the carbon atom.
Electron-accepting groups exert negative inductive effects to destabilize carbocation due to increase of electron deficit on the carbon atom. If the carbon atom with the vacant orbital is related to the atom in a state of sp2-hybridization or with a heteroatom which has a lone pair of electrons, carbocation is more stable due to the delocalization of charge on the conjugating system (р-π-conjugation or р-р-conjugation)
If the carbon atom with the vacant orbital is related to the atom in a state of sp2-hybridization or with a heteroatom which has a lone pair of electrons, carbocation is more stable due to the delocalization of charge on the conjugating system (р-π-conjugation or р-р-conjugation)

 Electron - accepting groups exert negative inductive effects to stabilize carboanion due to decrease of electron over on the carbon atom.
Electron - accepting groups exert negative inductive effects to stabilize carboanion due to decrease of electron over on the carbon atom.













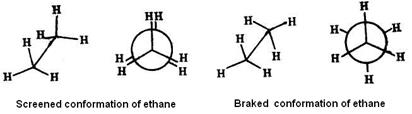

 Conformational isomers interconvert into each other without breaking of chemical bonds. Unlike structural isomers, different conformers can’t usually be isolated, because theyinterconverttoo rapidly. This kind of isomers can be fined by physical and chemical methods only.
Conformational isomers interconvert into each other without breaking of chemical bonds. Unlike structural isomers, different conformers can’t usually be isolated, because theyinterconverttoo rapidly. This kind of isomers can be fined by physical and chemical methods only. Rotation of a carbon atom of a C = C bond through an angle of 90° causes the breaking of the p bond.
Rotation of a carbon atom of a C = C bond through an angle of 90° causes the breaking of the p bond.

















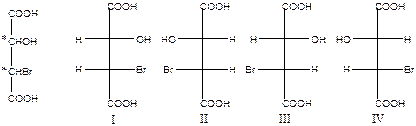
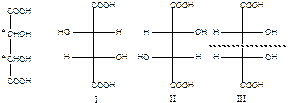 e.g.
e.g. а) rearrangement; b) condensation; c) addition; d) substitution;
а) rearrangement; b) condensation; c) addition; d) substitution; а) ЕS1; b) NA1; c) AN1; d) AE1; e) SN2; f) E; g) SE1.
а) ЕS1; b) NA1; c) AN1; d) AE1; e) SN2; f) E; g) SE1.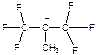 , 2)
, 2) 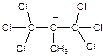 , 3)
, 3)  . Explain. а) 1,2,3; b) 3,1,2; c) 2,3,1; d) 1,3,2; e) 2,1,3; f) 3,2,1.
. Explain. а) 1,2,3; b) 3,1,2; c) 2,3,1; d) 1,3,2; e) 2,1,3; f) 3,2,1. а) chain isomerism; b) position isomerism; c) metamerism; d) tautomerism; e) functional group isomerism; f) enantiomerism; g) geometrical isomerism; h) conformational isomerism.
а) chain isomerism; b) position isomerism; c) metamerism; d) tautomerism; e) functional group isomerism; f) enantiomerism; g) geometrical isomerism; h) conformational isomerism. How many optical isomers has the following compound? In the following compound show chiral atoms as *. а) 2; b) 3; c) 4; d) 9; e) 8; f) compound has no optical isomers.
How many optical isomers has the following compound? In the following compound show chiral atoms as *. а) 2; b) 3; c) 4; d) 9; e) 8; f) compound has no optical isomers.