Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Translate the sentences below paying attention to the emphatic constructions.
Never before - никогда раньше ... не Example: Never before has this substance been obtained in such a pure form. Никогда раньше это вещество не было получено в таком чистом виде.
I. Never before has a rocket reached the Moon. 2. Never before did they admit the incompatibility of these requirements. 3. Never before did I attach importance to the destroying effect of corrosion. 4. Never before has the imagination of mankind been captivated (захватывать) so much by the concept of space.
Nor ... ( Neither) ... – а также не; к тому же не Example: Nor (Neither) was he capable of conceiving the contradiction. К тому же он не был способен понять, это противоречие.
1. Neither can a computer do any data processing without being instructed. 2. Nor should we be sure that in two successive observations we shall distinguish remarkable inconsistency with the theory. 3. Conventional methods of detection have some serious drawbacks. Nor are they effective in searching for new slates of particles. 4. The film "Is it Easy to Be Young?" proves that there cannot be, nor do there exist, specific youth problems. All of them reflect the processes developing in society as a whole. 5. Neither the protons nor the neutrons or electrons involved in the process of fission (деление) disappear. Nor do they become smaller. 6. Never before could we overcome experimental difficulties. Nor could we cope with (справиться с) the abundance of new information coming in every day.
TEXT 3 Electromagnetic Rest Mass
Before reading the text below answer the following question. What are the following scientists famous for? J. J. Thomson Oliver Heaviside George Frederick Charles Searle Wilhelm Wien Max Abraham Hendrik Lorentz For questions 1-7, read the text below and decide which answer (A, B, C or D) best fits each gap.
There were many (1)__________ in the 19th and the beginning of the 20th century - like those of J. J. Thomson (1881), Oliver Heaviside (1888), George Frederick Charles Searle (1896), - to understand how the mass of a(n) (2)__________ object varied with the velocity. Because the electromagnetic field carries part of the momentum of a moving charge, it was suspected that the mass of an electron would vary with velocity near the speed of light. Following Searle (1896), Wilhelm Wien (1900), Max Abraham (1902), and Hendrik Lorentz (1904) concluded that the velocity dependant electromagnetic mass of a body (3)_______ is m = (4 / 3)E / c2. According to them, this relation applies to the complete mass of bodies, because any form of inertial mass was considered to be of electromagnetic (4)________. Wien went on by stating, that if it is assumed that gravitation is an electromagnetic effect too, than there has to be a(n) (6)________ proportionality between (electromagnetic) inertial mass and (electromagnetic) gravitational mass. To explain the stability of the matter-electron configuration, Poincarй in 1906 introduced some sort of pressure of non-electrical nature, which contributes the amount − (1 / 3)E / c2 to the mass of the bodies, and (7)_________ the 4/3-factor vanishes.
1 A attempts B thoughts C ideas D works 2 A charged B loaded C inspired D forced 3 A at rest B in peace C in calmness D at repose 4 A origin B spring C root D basis 5 A origin B spring C root D basis 6 A strict B severe C austere D stringent 7 A therefore B naturally C moreover D even For questions 1-9, read the passage below. Use the word given in capitals at the end of the lines to form a word that fits in the same line. Albert Einstein
LISTENING
1. You are going to listen to the staff report “Joint Actions Helping to Bolster Nuclear Security China Links Up With IAEA on Nuclear Security for Summer Games “. Mind the proper names: Anita Nilsson, Director of the IAEA´s Office of Nuclear Security China Atomic Energy Authority (CAEA) the 2006 FIFA World Cup the 2007 Pan American Games Beijing 2. Speed listening. Note only the essential details of what you hear: 1. The Olympics………………………. 2. China and the IAEA…………………….. 3. A training workshop……………………….. 4. Anita Nilsson said……………………………… 5. IAEA´s work in Beijing……………………… 6. Chinese authorities………………………… 7. Nuclear security measures………………………………… 8. Help to Member states…………………………………………
3. General information: Complete the chart with the basic ideas:
4. Gap filling: Listen once again and complete the gaps in the summary of the passage below with the correct word or phrase you hear:
On the threshold of the Summer Olympic Games Chine and the IAEA are working together at strengthening _________________ and minimizing threats. For that purpose _______________ was held in Beijing. The IAEA’s role was to assist in integrating __________________into the existing Chinese security system. The spheres which are covered by the conducted advisory missions and training exercises are __________________. The IAEA has already rendered technical assistance to different international public events among which are ____________________. Member States of the IAEA get comprehensive help from the Office of Nuclear Security in form of __________________. This is vitally important for protecting ______________________ and fighting against___________________________.
5. Decide whether these statements are true, false or the information is not given:
1. China and the IAEA are creating country’s new security plan. 2. Work of Chine with the IAEA’s specialists has been lasting for one and a half year. 3. The IAEA’s work in Beijing is provided only for intelligence community. 4. A training workshop was widely covered in the press. 5. The China Atomic Energy Authority is the main coordinator of activities. 6. The IAEA is now actively working with Peru on arranging another training seminar for security officers.
7. Several Member States that need help in the formation of security planning have recently signed agreements with the IAEA. Work in pairs or groups. Discuss the topic mentioned in the staff report “Joint Actions Helping to Bolster Nuclear Security China Links Up With IAEA on Nuclear Security for Summer Games “. PRESENTATION Make up a presentation “MASS DEFECT” (See appendix 4) SECTION 4 BINDING ENERGY LEAD-IN Work in pairs or groups. Comment and discuss the statement below. The separate laws of Conservation of Mass and Conservation of Energy are not applied strictly on the nuclear level. It is possible to convert between mass and energy. Instead of two separate conservation laws, a single conservation law states that the sum of mass and energy is conserved. Mass does not magically appear and disappear at random. A decrease in mass will be accompanied by a corresponding increase in energy and vice versa. READING TEXT 1 Before reading the text below, work in small groups (2-3 students) and decide whether the statements 1-5 are true or false using your knowledge of the subject. Then read the text and check your guesses. a. Binding energy can not be considered equivalent to the mass defect. b. All the electrons are equally bound in the atom. c. Ionization is the process of removing an electron from an atom. d. Atom can stay in an excited state for a long time without changing its orbit. e. The only difference between x-rays and γ-rays is intensity of electromagnetic radiation. Overview of Binding Energy The loss in mass, or mass defect, is due to the conversion of mass lo binding energy when the nucleus is formed. Binding energy is defined as the amount of energy that must be supplied to a nucleus to completely separate its nuclear particles (nucleons). It can also be understood as the amount of energy that would be released if the nucleus was formed from the separate particles. Binding energy is the energy equivalent of the mass defect. Since the mass defect was converted lo binding energy (BE) when the nucleus was formed, it is possible to calculate the binding energy using a conversion factor derived by the mass-energy relationship from Einstein's Theory of Relativity. Einstein's famous equation relating mass and energy is E = mc² where с is the velocity of light (C = 2.998 x 10*8 m/sec). The energy equivalent of 1 amu can be determined by inserting this quantity of mass into Einstein's equation and applying conversion factors.
Conversion Factors:
Since 1 amu is equivalent to 931.5 MeV of energy, the binding energy can be calculated using Equation (1-2).
Example: Calculate the mass defect and binding energy for uranium-235. One uranium-235 atom has a mass of 235.043924 amu.
Solution: Step 1: Calculate the mass defect using Equation (1-1).
Step 2: Use the mass defect and Equation (1-2) to calculate the binding energy.
Energy Levels of Atoms: The electrons that circle the nucleus move in fairly well-defined orbits. Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88.000 eV is required to remove the innermost electron. The process of removing an electron from an atom is called ionization, and the energy required to remove the electron is called the ionization energy. In a neutral atom (number of electrons = Z) it is possible for the electrons to be in a variety of different orbits, each with a different energy level. The state of lowest energy is the one in which the atom is normally found and is called the ground state. When the atom possesses more energy than its ground state energy, it is said to be in an excited state.
Energy Levels of the Nucleus: The nucleons in the nucleus of an atom, like the electrons that circle the nucleus, exist in shells that correspond to energy states. The energy shells of the nucleus are less defined and less understood than those of the electrons. There is a state of lowest energy (the ground state) and discrete possible excited states for a nucleus. Where the discrete energy states for the electrons of an atom are measured in eV or keV, the energy levels of the nucleus are considerably greater and typically measured in MeV. A nucleus that is in the excited state will not remain at that energy level for an indefinite period. Like the electrons in an excited atom, the nucleons in an excited nucleus will transition towards their lowest energy configuration and in doing so emit a discrete bundle of electromagnetic radiation called a gamma ray (γ-ray). The only differences between x-rays and γ-rays are their energy levels and whether they are emitted from the electron shell or from the nucleus. The ground slate and the excited slates of a nucleus can be depicted in a nuclear energy-level diagram. The nuclear energy-level diagram consists of a slack of horizontal bars, one bar for each of the excited states of the nucleus. The vertical distance between the bar representing an excited state and the bar representing the ground state is proportional to the energy level of the excited state with respect to the ground slate. This difference in energy between the ground state and the excited state is called the excitation energy of the excited state. The ground state of a nuclide has zero excitation energy. The bars for the excited states are labeled with their respective energy levels. Figure 7 is the energy level diagram for nickel-60.
1.2. Find antonyms to the following words in the text above:
|
|||||||||||||||||||||
|
Последнее изменение этой страницы: 2016-08-01; просмотров: 329; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 18.221.235.209 (0.03 с.) |



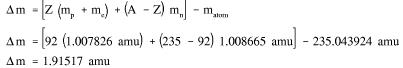
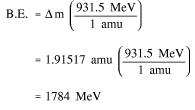
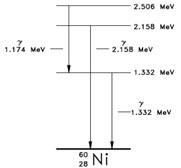 An atom cannot stay in the excited state for an indefinite period of time. An excited atom will eventually transition to either a lower-energy excited state, or directly to its ground state, by emitting a discrete bundle of electromagnetic energy called an x-ray. The energy of the x-ray will be equal to the difference between the energy levels of the atom and will typically range from several eV to 100.000 eV in magnitude.
An atom cannot stay in the excited state for an indefinite period of time. An excited atom will eventually transition to either a lower-energy excited state, or directly to its ground state, by emitting a discrete bundle of electromagnetic energy called an x-ray. The energy of the x-ray will be equal to the difference between the energy levels of the atom and will typically range from several eV to 100.000 eV in magnitude.