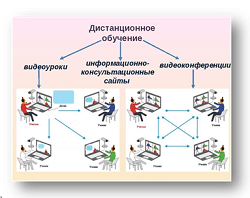
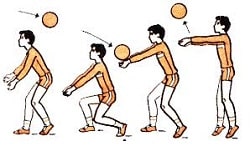
Заглавная страница Избранные статьи Случайная статья Познавательные статьи Новые добавления Обратная связь КАТЕГОРИИ: ТОП 10 на сайте Приготовление дезинфицирующих растворов различной концентрацииТехника нижней прямой подачи мяча. Франко-прусская война (причины и последствия) Организация работы процедурного кабинета Смысловое и механическое запоминание, их место и роль в усвоении знаний Коммуникативные барьеры и пути их преодоления Обработка изделий медицинского назначения многократного применения Образцы текста публицистического стиля Четыре типа изменения баланса Задачи с ответами для Всероссийской олимпиады по праву 
Мы поможем в написании ваших работ! ЗНАЕТЕ ЛИ ВЫ?
Влияние общества на человека
Приготовление дезинфицирующих растворов различной концентрации Практические работы по географии для 6 класса Организация работы процедурного кабинета Изменения в неживой природе осенью Уборка процедурного кабинета Сольфеджио. Все правила по сольфеджио Балочные системы. Определение реакций опор и моментов защемления |
Ex.5. Translate the following text.⇐ ПредыдущаяСтр 16 из 16
Catalyst. A catalyst is a substance that speeds up the rate of a chemical reaction but is not consumed during the course of the reaction. A catalyst will appear in the steps of a reaction mechanism, but it will not appear in the overall chemical reaction (as it is not a reactant or product). Generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. By lowering the activation energy, the rate constant is greatly increased (at the same temperature) relative to the uncatalyzed reaction. There are many types of catalysts in the world. Many reactions are catalyzed at the surface of metals. In biochemistry, enormous numbers of reactions are catalyzed by enzymes. Catalysts can either be in the same phase as the chemical reactants or in a distinct phase. Catalysts in the same phase are called homogeneous catalysts, while those in different phases are called heterogeneous catalysts. For example, if we have Pt metal as a catalyst for the reaction of hydrogen gas and ethene gas, then the Pt is a heterogeneous catalyst. However, an enzyme in solution catalyzing a solution phase biochemical reaction is a homogeneous catalyst. Another important idea about catalysts is that they are selective. That is the catalyst doesn't just speed up all reactions, but only a very particular reaction. This is the key to many chemical transformations. When you only want to perform a particular chemical change, you look for a catalyst that will speed up that specific reaction but not others. Enzymes are remarkable in this way. Living biological systems require a myriad of specific chemical transformations and there is a unique enzyme to catalyze each of them. Effect of catalysts. The effect of a catalyst is that it lowers the activation energy for a reaction. Generally, this happens because the catalyst changes the way the reaction happens (the mechanism). We can visualize this for a simple reaction coordinate in the following way. In a more generally sense, the catalyzed reaction may have a number of new barriers and intermediates. However, the highest barrier will now be significantly lower than the previous largest barrier. UNIT XIX BIOACTIVE COMPOUNDS AND BIOCHEMISTRY A bioactive compound is a compound that has an effect on a living organism, tissue or cell. In the field of nutrition bioactive compounds are distinguished from essential nutrients. While nutrients are essential to the sustainability of a body, the bioactive compounds are not essential since the body can function properly without them, or because nutrients fulfil the same function. Bioactive compounds can have an influence on health. Bioactive compounds are found in both plant and animal products or can be synthetically produced. Examples of plant bioactive compounds are carotenoids and polyphenols (from fruits and vegetables), or phytosterols (from oils). Example in animal products are fatty acids, found in milk and fish. Some examples of bioactive compounds are flavonoids, caffeine, carotenoids, carnitine, choline, coenzyme Q, creatine, phytosterols, polysaccharides, glucosinolates, polyphenols, anthocyanins prebiotics, taurine and others. There are two biggest classes of bioactive compounds. They are hormones and vitamins. Hormones Hormones are organic substances secreted by plants and animals. They are special chemical messengers in the body that are created in the endocrine glands. These messengers control most major bodily functions, e.g. such as digestion, metabolism, respiration, tissue function, sensory perception, sleep, excretion, lactation, stress, growth and development, movement, reproduction, and even mood. Hormones affect distant cells by binding to specific receptor proteins in the target cell resulting in a change in cell function. Hormone synthesis may occur in specific tissues of endocrine glands or in other specialized cells, which are part of the endocrine system. Hormone synthesis occurs in response to specific biochemical signals induced by a wide range of regulatory systems. The main hormone-producing glands are: hypothalamus being responsible for body temperature, hunger, moods and the release of hormones from other glands and controlling thirst, sleep and sex drive; parathyroid controlling the amount of calcium in the body; thymus, playing a role in the function of the adaptive immune system and the maturity of the thymus and producing T-cells; p ancreas producing the insulin that helps control blood sugar levels; thyroid producing hormones associated with calorie burning and heart rate; adrenal producing the hormones that control sex drive and cortisol, the stress hormone; pituitary controlling other glands; pineal, a lso called the thalamus, producing serotonin derivatives of melatonin, which affects sleep; ovaries, only in women, secreting estrogen, testosterone and progesterone, the female sex hormones; testes, only in men, producing the male sex hormone, testosterone, and produce sperm. These glands work together to create and manage the body's major hormones.
Hormones have diverse chemical structures, mainly of 3 classes: eicosanoids, steroids, and amino acid derivatives (amines, peptides, and proteins). Major Types of Hormones The body has many different hormones, but certain types have a bigger role to play in the body's health and well-being. Understanding these roles is important for those looking to protect and manage their health. We would like to mention some of them: Cortisol has been called the "stress hormone" because of the way it assists the body in responding to stress. This is just one of several functions of this important hormone. Adrenaline triggers the body's fight-or-flight response. This reaction causes air passages to dilate to provide the muscles with the oxygen they need to either fight danger or flee. Adrenaline also triggers the blood vessels to contract to re-direct blood toward major muscle groups, including the heart and lungs. The body's ability to feel pain also decreases as a result of adrenaline, which is why you can continue running from or fighting danger even when injured. Adrenaline causes a noticeable increase in strength and performance, as well as heightened awareness, in stressful times. After the stress has subsided, adrenaline’s effect can last for up to an hour. Thyroxine plays a crucial role in heart and digestive function, metabolism, brain development, bone health and muscle control. It affects almost all of the body's systems, which means proper thyroxine levels are vital for health. Melatonin (“sleep hormone”) is essential to signaling the relaxation and lower body temperature that help with restful sleep. Levels of melatonin are higher at night, signaling the body that it is time to rest. Melatonin levels change throughout the day Insulin allows the cells in the muscles, fat and liver to absorb glucose that is in the blood. Insulin production and release is a tightly regulated process, allowing the body to balance its metabolic needs. Vitamins The value of eating a certain food to maintain health was recognized long before vitamins were identified. The ancient Egyptians knew that feeding liver to a person would help cure night blindness, an illness now known to be caused by a vitamin A deficiency. The advancement of ocean voyages during the Renaissance resulted in prolonged periods without access to fresh fruits and vegetables, and made illnesses from vitamin deficiency common among ships' crews. In 1747, the Scottish surgeon James Lind discovered that citrus foods helped prevent scurvy, a particularly deadly disease in which collagen is not properly formed, causing poor wound healing, bleeding of the gums, severe pain, and death. The term “vitamine” appeared in 1912. It consisted of two words “vital” and “amine”. However, in 1920, it was determined that newly discovered vitamin C did not contain amine component and the letter “e” was removed from the name.
Vitamins may be defined as organic substances that play a required catalytic role within the cell (usually as components of coenzymes or other groups associated with enzymes) and must be obtained in limited amounts through the diet. Vitamin requirements are specific for each organism, and their deficiency may cause disease. Vitamins are classified as either water-soluble or fat-soluble. In humans there are 13 vitamins: 4 fat-soluble (A, D, E, and K) and 9 water-soluble (8 B vitamins and vitamin C). Many types of water-soluble vitamins are synthesized by bacteria. Fat-soluble vitamins are absorbed through the intestinal tract with the help of lipids (fats). Because they are more likely to accumulate in the body, they are more likely to lead to hypervitaminosis than are water-soluble vitamins. Fat-soluble vitamin regulation is of particular significance in cystic fibrosis. Although a vitamin is usually defined as an organic chemical which an animal or human must obtain from the diet, this is not entirely true. There are some examples. ВVitamin A does not occur in the plant kingdom, but the pigment carotene is universally present in green plants, and most animals can split a molecule of carotene into two molecules of vitamin A. The exceptions are cats and probably other carnivores, which under natural conditions have to obtain the preformed vitamin by consuming the tissues of other animals. Niacin, too, is not an absolute requirement, since most animals (cats again being an exception) can synthesize it from the amino acid tryptophan if the latter is present in excess of its use for protein synthesis. Vitamin D is not a true vitamin: most species do not need it in their diet, because they obtain an adequate supply through the exposure of skin to sunlight, which converts a sterol present in dermal tissue to vitamin D. The vitamin is subsequently metabolized to form a hormone that acts to control the absorption and utilization of calcium and phosphate. Animals such as rodents, which normally have little exposure to sunlight and search for food mostly at night, appear to have evolved so as to be independent of vitamin D so long as their intakes of calcium and phosphate are well-balanced. Vitamin C (ascorbic acid) is an essential chemical in the tissues of all species, but most can make it for themselves, so that for them it is not a vitamin. Presumably, species that cannot synthesize vitamin C—they include humans, guinea pigs, and fruit-eating bats—had ancestors that lost the ability at a time when their diet was rich in ascorbic acid. Bacteria vary greatly in their need for vitamins. Many are entirely independent of outside sources, but at the other extreme some of the strains of bacteria found in milk (i.e., Lactobacillus) have lost the ability to synthesize the B vitamins that they need. This property has made them useful for assaying extracts of foods for their vitamin B content. Indeed, many vitamins of this group were first discovered as growth factors for bacteria before being tested with animals and humans. The mixed bacterial flora in the guts of animals are, on balance, synthesizers of the B vitamins. For one B vitamin—cobalamin, or vitamin B12—bacterial fermentation is the only source, though it can be obtained indirectly from the tissues or milk of animals that have obtained it themselves from bacteria. The generalization that “the animal kingdom lives on the plant kingdom” is therefore not the whole truth, because animals rely partly on bacteria for this one micronutrient. Sellers of bioactive substances often attribute health benefits to these compounds, but there is insufficient research into the effectiveness and safety of these substances, either in long term use or in quantities that exceed normal consumption levels. In addition, some flavonoids have been shown to influence the effects of drugs. However, a number of bioactive substances have been shown to act as antioxidants. As bioactive compounds are not essential, advice on daily intake is often unregulated. Biochemistry Biochemistry is the branch of science that explores the chemical processes within and related to living organisms. It is a laboratory based science that brings together biology and chemistry. By using chemical knowledge and techniques, biochemists can understand and solve biological problems. Biochemistry focuses on processes happening at a molecular level. It focuses on what’s happening inside our cells, studying components like proteins, lipids and organelles. It also looks at how cells communicate with each other, for example during growth or fighting illness. Biochemists need to understand how the structure of a molecule relates to its function, allowing them to predict how molecules will interact. ВBiochemistry covers a range of scientific disciplines, including genetics, microbiology, forensics, plant science and medicine. Because of its breadth, biochemistry is very important and advances in this field of science over the past 100 years have been staggering. It’s a very exciting time to be part of this fascinating area of study. Biochemists В· Provide new ideas and experiments to understand how life works; В· Support our understanding of health and disease; В· Contribute innovative information to the technology revolution; В· Work alongside chemists, physicists, healthcare professionals, policy makers, engineers and many more professionals.
Methods in biochemistry Like other sciences, biochemistry aims at quantifying, or measuring, results, sometimes with sophisticated instrumentation. The earliest approach to a study of the events in a living organism was an analysis of the materials entering an organism (foods, oxygen) and those leaving (excretion products, carbon dioxide). This is still the basis of so-called balance experiments conducted on animals, in which, for example, both foods and excreta are thoroughly analyzed. For this purpose many chemical methods involving specific colour reactions have been developed, requiring spectrum-analyzing instruments (spectrophotometers) for quantitative measurement. Gasometric techniques are those commonly used for measurements of oxygen and carbon dioxide, yielding respiratory quotients (the ratio of carbon dioxide to oxygen). Somewhat more detail has been gained by determining the quantities of substances entering and leaving a given organ and also by incubating slices of a tissue in a physiological medium outside the body and analyzing the changes that occur in the medium. Because these techniques yield an overall picture of metabolic capacities, it became necessary to disrupt cellular structure (homogenization) and to isolate the individual parts of the cell—nuclei, mitochondria, lysosomes, ribosomes, membranes—and finally the various enzymes and discrete chemical substances of the cell in an attempt to understand the chemistry of life more fully. REVISION EXERCISES Ex.1. Answer the following questions: 1. What is a bioactive compound? 2. Where bioactive compounds can be found? 3. What are the biggest classes of bioactive compounds? 4. Where may hormone synthesis occur in? 5. What are the main hormone-producing glands? 6. How can vitamins be classified? 7. Do bioactive substances benefit human health? 8. What is biochemistry? 9. What are the main purposes of biochemistry? 10. What are the most widely used methods of biochemistry? Ex.2. Match the words with their definitions:
Ex.3. Say whether the following statements are true or false: 1. Bioactive compounds cannot be synthetized; they should be obtained from the diet. 2. Bioactive compounds are important just for the metabolic processes; they don’t have an influence on health. 3. Hormones control main functions of the body, even your mood depend on them. 4. The endocrine glands of the organismВ operate as one team. 5. Vitamin requirements are general for all people. 6. The more vitamins people take, the better it is for their health. 7. By using chemical knowledge and techniques, biochemists can understand and solve different problems of inorganic chemistry. Ex.4. Insert the necessary word: 1. Biochemistry is the study of the chemistry of …. 2. It is a vast and exciting field in which important discoveries about how life is maintained and how В… occur are being made every day. 3. In particular, there has been rapid growth in the understanding of how living cells manufacture and В… necessary for life. 4. This not only has been beneficial for detection and В… but also has spawned a new field— …, which uses nature’s “machinery” to synthesize desired substances. 5. Many other products, including …, are also being produced by the techniques of biotechnology. 6. An understanding of biochemistry also allows our society to produce …. (treatment of diseases, healthier processed foods, living systems, use the molecules, biotechnology, diseases, natural pesticides)
|
|||||||||||||||||||||||
|
Последнее изменение этой страницы: 2017-01-27; просмотров: 441; Нарушение авторского права страницы; Мы поможем в написании вашей работы! infopedia.su Все материалы представленные на сайте исключительно с целью ознакомления читателями и не преследуют коммерческих целей или нарушение авторских прав. Обратная связь - 3.135.205.146 (0.017 с.) |